Building a Scalable Cochlear Implant Program
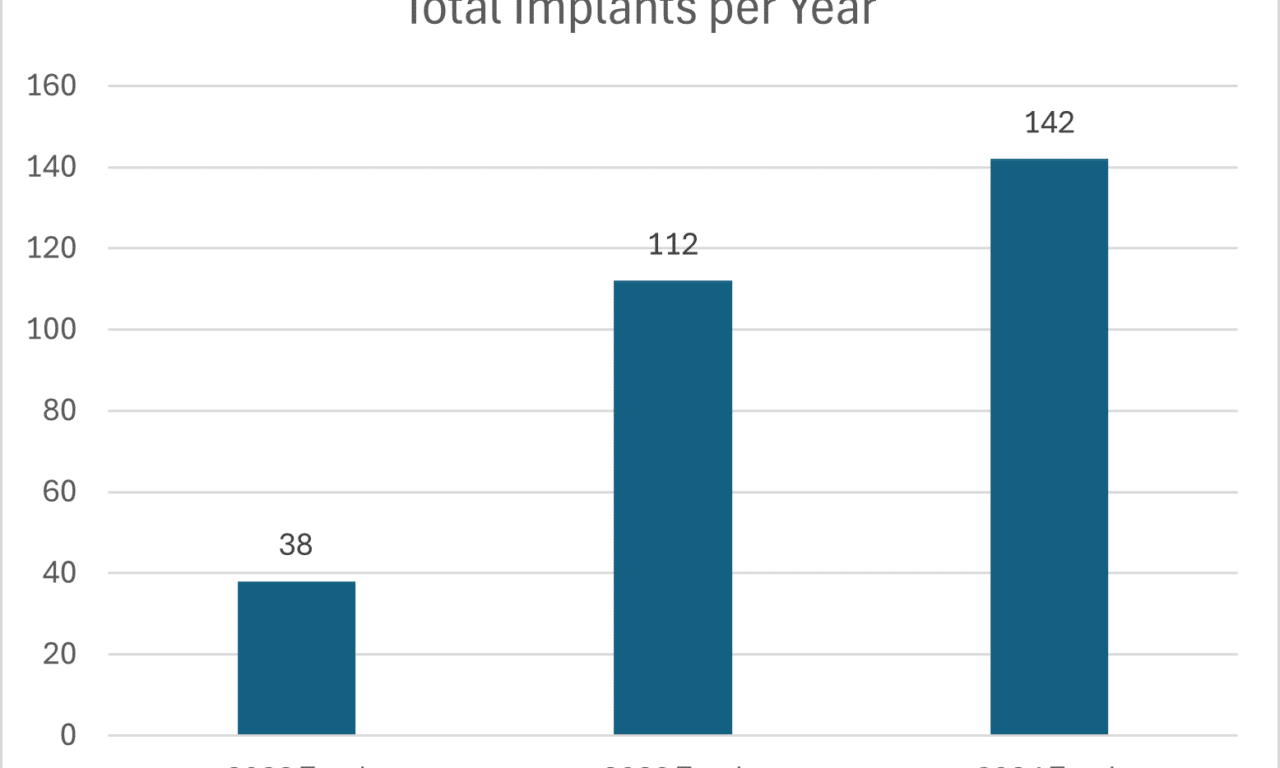
A cochlear implant (CI) program in South Carolina has demonstrated how strategic workflow design, data-driven efficiency measures, and team-based care can dramatically expand access, improve patient satisfaction, and sustain rapid program growth in an underserved region.