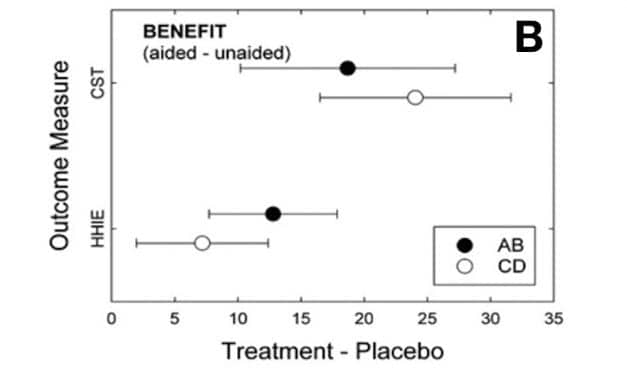
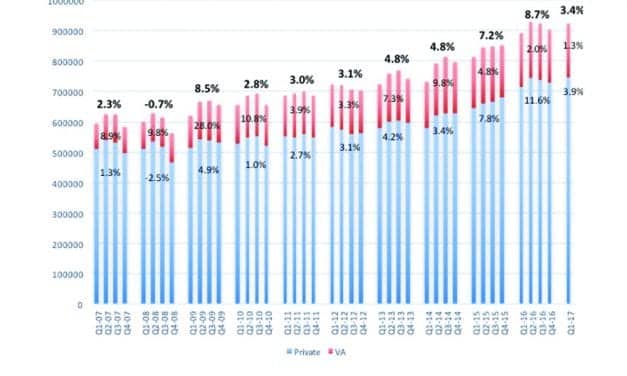
HLAA Announces 2017 Walk4Hearing Events
The Hearing Loss Association of America (HLAA)—an organization dedicated to educating, advocating, and supporting individuals with hearing loss—is thrilled to announce its 20 Walk4Hearing events across the nation this year, including two new locations in Buffalo, NY and Louisville, KY.
Read More