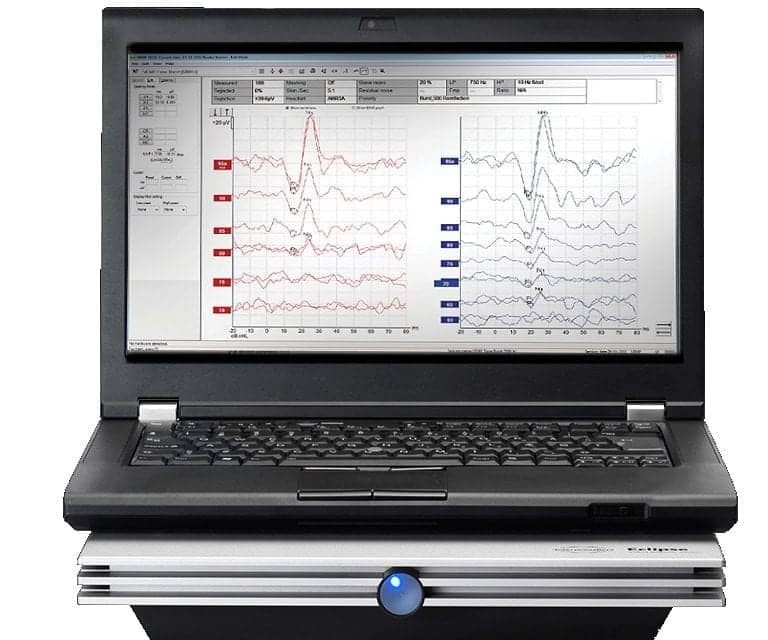
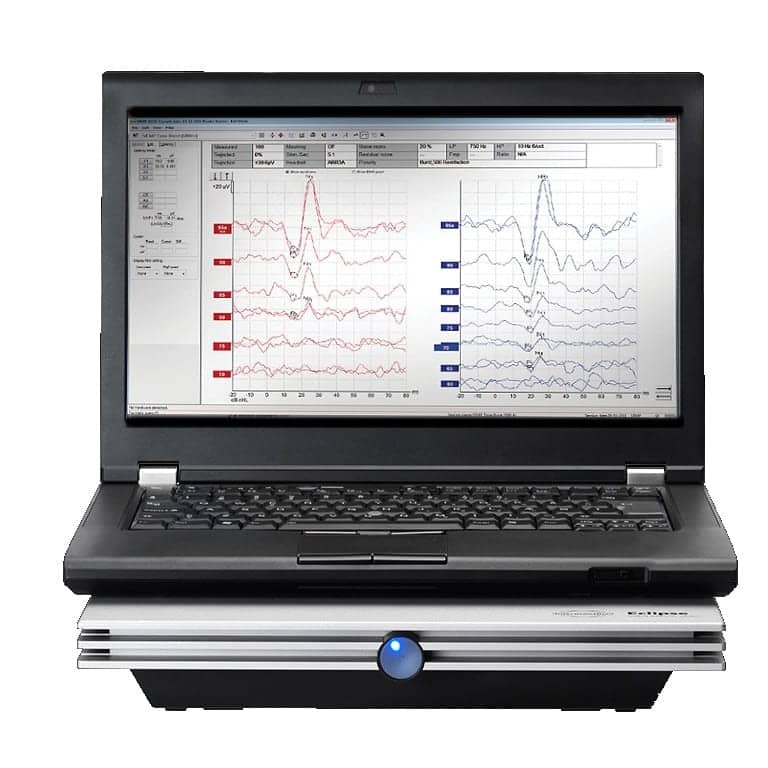
Interacoustics has received FDA approval for vestibular evoked myogenic potential (VEMP) testing with their Eclipse hardware, the company announced. Interacoustics can now offer clinicians in the United States technology that has been widely tested and well received by clinicians globally.
The VEMP tests measure and analyze the vestibular evoked myogenic potential generated by loud stimuli. The Eclipse comes with pre-programmed cVEMP & oVEMP protocols.
“This approval from FDA is of great value to US clinicians who now have the possibility to conduct comprehensive vestibular investigations for their patients,” said Interacoustics US Director of Clinical Audiology Cammy Bahner, AuD. “With Interacoustics’ unique Eclipse patient monitor, clinicians can measure if the patient has a correct muscle tonus throughout the entire VEMP assessment. Also, the Eclipse VEMP ensures a confident and reliable result by applying EMG-based scaling of the recorded waveforms.”
The Eclipse VEMP can stimulate up to 100 dB nHL with clicks, 250 Hz, 500 Hz, 1 kHz, 2 kHz, and 4 kHz tone bursts, and Interacoustics says the addition of this valuable diagnostic test will provide excellent new opportunities for its users. “It is evident that users can combine the VEMP test with the Eclipse EP25 test for the most comprehensive electrophysiological assessment available,” says Interacoustics US Director Richard Mitchell.
For details, visit the Interacoustics website.
Source: Interacoustics