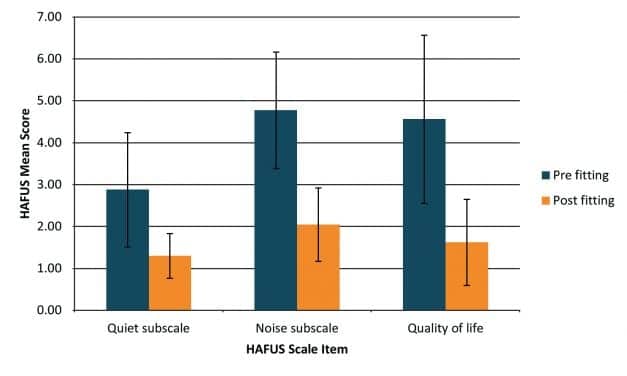
A Perspective on the Indiana University OTC Outcome Study
There is much to be learned from the recent IU study on OTC hearing aids. However, as its authors state, the findings are specific to that study and should not be generalized to the population of hearing-impaired individuals at large. A comparison is offered here to an audiology clinic using best practices. Results indicate that removing the professional from the fitting and follow-up process yields significantly lower levels of benefit, satisfaction, and use. If OTC hearing aids become a reality, this should be clearly stated on advertising and packaging; consumers should be informed that research has shown 45% of individuals returned hearing aids that were self-fit. Improving accessibility and affordability is only worthwhile if use and satisfaction are not sacrificed in the process.
Read More