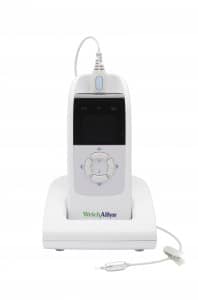
Welch Allyn, Skaneateles Falls, NY, a leading medical diagnostic device company, has introduced its next-generation OAE Hearing Screener, which is designed as a fast, objective pediatric hearing testing device that can be administered at the push of a button.
Designed to detect hearing loss in newborns, infants, toddlers, pre-school, and school-age children, the device does not require patient response or practitioner interpretation of exam results. The rechargeable, portable device features wireless Bluetooth® connectivity that enables automated patient data transfer to Data Manager software that lets users sort data and generate reports.
“The new Welch Allyn OAE Hearing Screener gives providers a fast, accurate, objective response so their patient doesn’t have to,” said Rick Farchione, senior global category manager at Welch Allyn in a press statement. “But this new hearing screener is more than a state-of-the-art diagnostic device—it’s a connected solution for healthcare professionals who will now be able to enroll even their youngest patients in early intervention programs to help prevent language disabilities before they occur. In a matter of seconds, it provides data that the clinician can use to help improve patient outcomes.”
The compact Welch Allyn OAE Hearing Screener presents a soft pair of tones through a probe placed in the patient’s ear canal. A small microphone in the probe measures the OAE and compares the response to normal data—assigning a “pass” or “refer” result to the screening on an intuitive full-color display. The presence of “passing” OAEs is highly correlated with normal hearing and middle ear function.
The Joint Committee on Infant Hearing (JCIH) 2000 recommends universal screening of hearing loss before hospital discharge. The JCIH 2000 statement promotes a system as essential for the diagnosis and intervention of early hearing loss. That system is composed of screening before hospital discharge, follow-up, and diagnosis for infants needing additional care and the intervention and habilitation for infants identified with hearing loss.
“Significant hearing loss can develop after the newborn period, so screening at birth is important, but it is not enough,” added Farchione. “Regular hearing screening is important throughout childhood. The availability of a fast, accurate and objective screening device can help pediatricians perform tests on younger patients, potentially increasing the number of at risk patients that are referred to a hearing specialist for further evaluation and treatment.”
The OAE Hearing Screener can be fitted with a variety of disposable ear-tip sizes including newborn, infant, toddler, and adolescent. It features on-screen prompts and in-the-ear calibration and comes equipped with patented noise-canceling technology to help eliminate poor test results due to ambient sound. Features include data storage for up to 250 tests, a charging cradle, and a Bluetooth® thermal printer that can provide graphic and tabular reports in seconds.
For more information, visit: www.welchallyn.com/oae.
Source: Welch Allyn