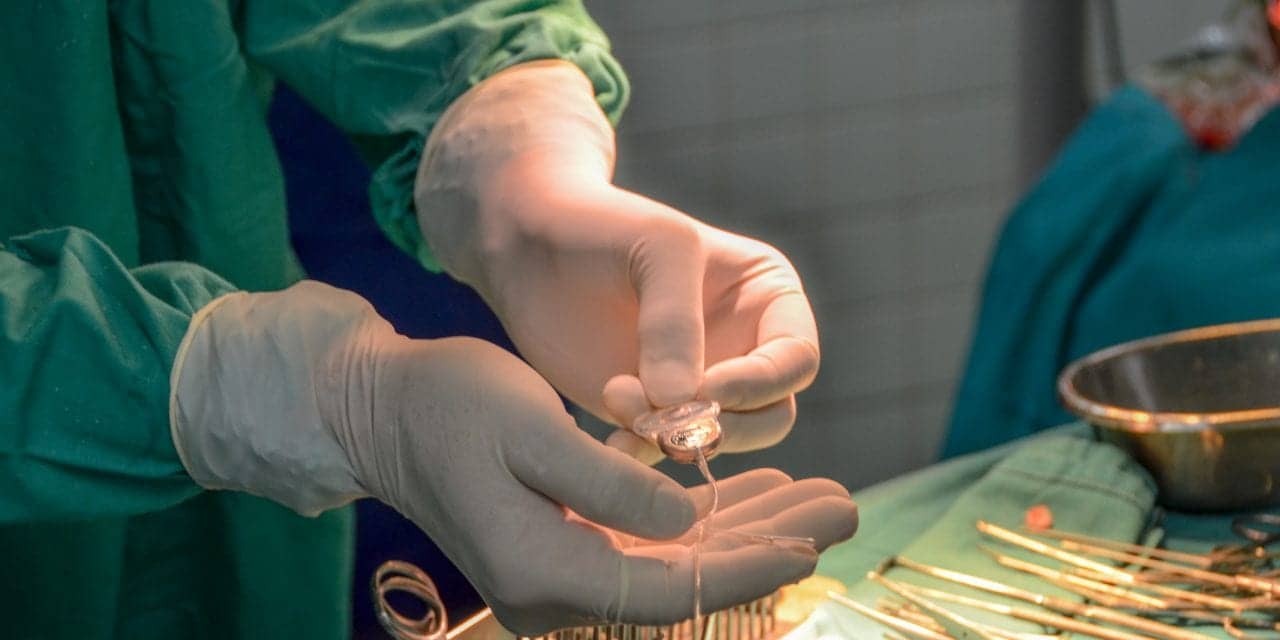
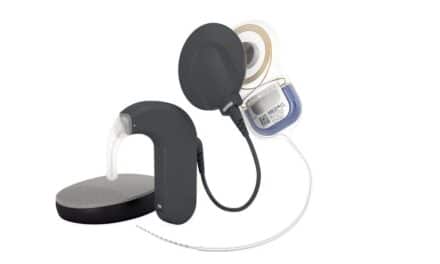
Envoy Medical Corporation, a hearing health company focused on providing innovative technologies across the hearing loss spectrum, announced the first patient has been enrolled and implanted in the Envoy Acclaim Cochlear Implant early feasibility study at Mayo Clinic in Rochester, Minn. This surgery marks a “key milestone” for Envoy Medical on the road toward bringing the Acclaim Cochlear Implant to market, according to the company’s announcement. If approved by the FDA, the Acclaim would be the “first-of-its-kind cochlear implant designed to be fully implanted and use the ear, rather than a microphone, to pick up sound.”

The implantation surgery was conducted by Colin Driscoll, MD, practicing neurotologist, professor of otolaryngology – head and neck surgery at Mayo Clinic, and principal investigator for this study. After a healing period, the Acclaim Cochlear Implant will be activated by Aniket Saoji, PhD, associate professor of otolaryngology – head and neck surgery at Mayo Clinic and co-investigator of the study. Both investigators serve on Envoy Medical’s Cochlear Implant Advisory Board.
“We at Envoy Medical are extremely proud that our Acclaim early feasibility study is taking place at Mayo Clinic. There is a strong medical device history and culture here in Minnesota, and we are honored to add to that legacy,” said Brent Lucas, CEO of Envoy Medical. “This study is an important step towards demonstrating whether the fully implanted Acclaim Cochlear Implant works as it was designed, turning the page to a new chapter in cochlear implants. We hope to change the status quo within the hearing industry, and I am so proud of our team for bringing this breakthrough technology to fruition—implantable medical devices are not for the faint of heart.”
Based on an Investigational Device Exemption (IDE) granted by the US Food and Drug Administration (FDA), the Envoy Acclaim early feasibility study is expected to last 18 months and will be followed by a pivotal trial to support a Premarket Approval (PMA) application to the FDA.
Patients interested in learning more about the study should contact Amy Pajula, customer experience manager, at [email protected] for more information.
About the Fully Implanted Acclaim® Cochlear Implant
The Acclaim Cochlear Implant is designed to leverage “unique sensor technology from the fully implanted Esteem active middle ear implant.” Intended to address the limitations of current microphone-based hearing devices, Envoy Medical’s fully implanted technology includes “a completely unique sensor designed to leverage the natural anatomy of the ear instead of a microphone to capture sound.” The Esteem was FDA-approved in 2010.
The Acclaim Cochlear Implant received the Breakthrough Device Designation from the FDA. If approved by the FDA, the Acclaim would be the ”first-of-its-kind cochlear implant designed without any externally worn components and to use the ear to pick up sound. ”
CAUTION The fully implanted Acclaim Cochlear Implant is an investigational device. Limited by United States law to investigational use.
Important safety information for the Esteem can be found at: https://www.envoymedical.com/safety-information.
Source: Envoy Medical Corporation