Technology that obviates the need for sedating children
Physiological artifacts, primarily of myoelectric origin, often require patient sedation for an auditory brainstem response (ABR) test in pediatric patients. Sedation imposes health risks and is counterindicated in many patients, thus limiting ABR application. Even when ABR is administered under anesthesia, electromagnetic interference (EMI), which is always present in the operating room, contaminates responses. EMI often forces ABR tests to be administered in a shielded room, but such rooms are costly, require space, and are often unavailable.
Integrity™, a new wireless auditory evoked potential (AEP) and otoacoustic emissions (OAE) analyzer, employs a range of technologies that enable AEP testing in nonsedated patients and virtually any clinical environment. Eliminating sedation from ABR tests dramatically reduces patient risk, the cost of administering the test, and broadens the patient population that can be tested. Thus, AEPs are becoming practical, and results attainable from virtually any patient, elevating the level of patient care.
Auditory Brainstem Response
ABR is one of the smallest AEPs. It is elicited by short-duration stimuli such as a wide-band click or a frequency-specific tone burst, recorded from patient-mounted electrodes. Normal ABR has characteristic wave morphology—five consecutive peaks (Waves I to V) appearing between 1 and 6 milliseconds after the stimulus at higher stimulus levels and somewhat later at lower levels.
The latencies of ABR Waves I, III, and V and interpeak intervals between them have the largest diagnostic value, and, therefore, need to be measured with maximum precision in a diagnostic test.1-3 Of those peaks, Wave V has the largest amplitude and remains as the stimulus level diminishes to near threshold, which explains its application for hearing threshold estimation.
ABR is a well-established clinical test for differential diagnostics of cochlear versus retro-cochlear pathology, particularly “auditory neuropathy” (auditory dys-synchrony), newborn hearing screening. It is particularly important for diagnostics and estimating hearing thresholds in infants and young children.1,2,4
Muscular Artifacts and the Need for Sedation
Muscular activity generates very strong electrical artifacts, 100-200 uV RMS and higher, which interfere with the much smaller ABR. They are always present in nonrelaxed (nonsedated) patients and normally diminish in sleep, particularly under sedation. Yet, some muscles in relaxed and even sedated patients may move and generate artifacts. In some patients, myogenic artifacts may be present even without visible body movements. The frequencies of muscle artifacts range from 50 Hz-500 Hz (ie, within the ABR frequency range 30 Hz to 3,000 Hz), and therefore, they freely pass through the ABR band-pass filter. The only way of coping with muscle artifacts is digital averaging a number of repeated responses, or sweeps.
The effect of muscle and eye artifacts on the average is somewhat reduced by artifact rejection (ie, rejecting the sweeps containing artifacts greater than a certain preset value called artifact-rejection threshold). Accepted sweeps are averaged to further reduce noise, but averaging still does not produce clear responses in the presence of muscular artifacts, because even the sweeps with the amplitude below ART are contaminated with noise. Therefore, with conventional ABR equipment, the patient must be “quiet” (lying down, relaxed, eyes closed, asleep), and pediatric patients are often sedated or anesthetized.
Very young infants (up to 2 to 3 months of age) may sleep most of the time required for an ABR test, and myogenic artifacts are rarely an issue in such patients. However, sedation or anesthesia is almost always required for older infants and children from 3 months to 4 to 5 years of age to control behavior and eliminate muscular motion artifacts.5 A sedative, such as chloral hydrate, or a general anesthetic is administered. In some hospitals, this necessitates the use of an operating room; in others, sedation may be administered in an outpatient suite. In either case, sedation or anesthesia requires continuous monitoring and specialized personnel with training in cardiopulmonary life support.
The American Academy of Pediatrics (AAP) has recently updated its guidelines for sedation for therapeutic and diagnostic procedures.6 According to the guidelines, sedation of pediatric patients has serious associated risks, such as hypoventilation, apnea, airway obstruction, laryngospasm, and cardiopulmonary impairment. To mitigate the risks associated with sedation, the new AAP guidelines recommend a comprehensive checklist that includes medical supervision, careful presedation evaluation for underlying medical or surgical conditions, appropriate patient fasting, clear understanding of the pharmacokinetic and pharmacodynamic effects of the sedatives, appropriate airway management (including having size-appropriate equipment) to allow rescue of the patient should there be an adverse response, sufficient numbers of staff to both carry out the procedure and monitor the patient, appropriate physiologic monitoring during and after the procedure, and stipulations concerning the recovery process of the patient.
Logistically, scheduling and performing all of these procedures may be quite challenging and time-consuming for the clinician, but more importantly, there are conditions that may require additional considerations or counterindicate sedation, or sedation facilities may not be available for the clinician administering ABR. In many cases, follow-up tests are required to monitor hearing condition over time, and multiple sedations would be required, but may not be indicated. In some cases, parents do not consent to sedating their child for a hearing test, preventing the administration of an ABR test. If nonsedated ABR results are unattainable, the patient’s hearing function may not be properly assessed and the patient may not receive appropriate care.
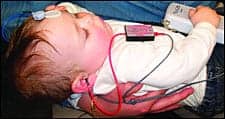
|
| FIGURE 1. Placement of the VivoLink™ on an infant with mother (top) and in a toddler’s toy backpack (bottom). Inset: Amplitrode® with the non-inverting (+) and inverting (-) electrode clips. Color-coded release buttons allow easy snap-on and snap-off of the electrodes. |
Thus, the need for sedation increases the risk to the patient, the costs of the test, and liability for the institution and examiner, and reduces clinical applicability of ABR and the level of patient care.
Extraneous Noises
Multiple extraneous noises affect ABR recording: conducted electric, electromagnetic field-induced, and radio frequency (RF) noises. Conducted noises come from AC-power lines through the power cord and computer interface cable. Electromagnetic interference (EMI) originating from surrounding electrical wiring and equipment, which exists in any clinical environment, is especially strong in operating rooms,5 intensive care units (ICU), neonatal ICUs (NICU), hospital wards, and some doctors’ offices. In some instances, EMI may be so strong that it can make ABR testing very difficult or even impossible. To reduce EMI, electromagnetically shielded rooms (Faraday cages) are used in some clinics. However, Faraday cages are expensive ($25,000 to $40,000), take up space, and may not be available where an ABR test is required. For example, they cannot be installed in the NICU, ICU, operating rooms, and smaller offices. Even where available, they complicate the logistics and limit the time available for administering ABR.
New Technologies
Integrity™ is the first wireless diagnostic AEP and OAE analyzer. It employs three novel technologies:
In situ amplification. The Amplitrode® (Figure 1), a patented miniature AEP amplifier, snaps directly onto the AEP electrode,6 and AEP signals are amplified in-situ (ie, right “on the site” of their appearance on the skin surface). This dramatically reduces electric, magnetic, and RF field-induced interferences, yielding a clear signal even in electromagnetically noisy environments like OR, NICU, and ICU.

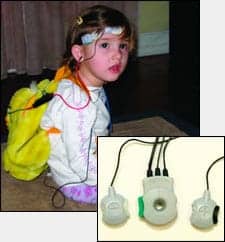
|
| FIGURE 2. Placement of the VivoLink™ on a lanyard. The VivoLink sends the information wirelessly to the computer. |
Wireless recording. Any PC-based ABR system contains a “box” connected to a PC via a serial or USB interface cable. The cable may introduce electrically conducted noise from the power line, even when testing in a Faraday cage. In the Integrity, communication between the video cassette–sized “box,” VivoLink™ (Figure 2), and computer occurs through wireless communication that completely eliminates the electrical path between the computer and AEP amplifier, and thus the introduction of conducted noise. Also, wireless communication provides exceptional electrical safety to the patient and the convenience of testing from a distance. For example, when testing infants, VivoLink can be placed in an infant’s crib, bassinet, incubator, stroller, or car seat; when testing toddlers, it can be placed in a toy backpack; with adults, a lanyard can be used. The PC can be placed anywhere within a 30-foot (10-meter) distance.
Kalman-weighted averaging (filtering). To reduce the effect of EMG artifacts in conventional ABR systems, many clinicians utilize the “Pause/Resume” buttons on the user interface. During testing, they carefully watch the “On-going EEG” display and press “Pause” when the noise is high, and “Resume” when the noise goes lower. This can be time-consuming for the clinician. The Integrity employs a patented technique based on a signal-processing method known as the Linear Minimum Mean-Square Error Filter, or Kalman filter.7,8 The Kalman Filter optimally weights each of the individual sweeps to obtain an ABR with minimum probability of error, regardless of the patient’s muscular activity.9 Clinically, ABR test results are attainable even in the presence of the patient’s muscular activity, thus removing the need for relaxation, sleep, or sedation.
Clinical ABR Experiments
Numerous experiments with Integrity ABR have been conducted in nonsedated and often active subjects—infants, young children, and adults—in various electromagnetic environments. In the experiments, ABRs were recorded under various combinations of subject and environmental conditions, from sleeping to awake, inactive and active (eg, chewing, drinking water, walking, talking, etc), which included electric and magnetic field strengths from very “quiet” (less than 0.1 V/m and 0.1 mG, respectively) to “very noisy” (70 V/m and 40 mG, substantially exceeding those found in clinical environments where ABR could be administered). Under all experimental conditions, ABRs were attainable, with no statistical difference in the latencies of ABR waves or thresholds. It should be noted that recording in active subjects required a larger number of sweeps to obtain results than in relaxed subjects.
Does it Work in Real Life?
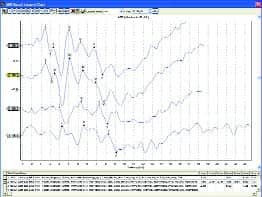
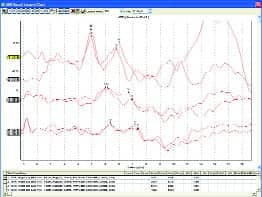
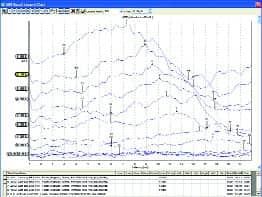
|
| FIGURE 3. Top: 10-week old infant in NICU. Middle: 4-month old intermittently crying and sucking on pacifier. Bottom: Non-cooperative adult (500 Hz tone burst). |
Clinicians know that sometimes new techniques work well in a research lab, but not as well in the “real clinical world.” Integrity ABR was first introduced in early 2006 and has been installed and used in dozens of clinical facilities. They include university clinics, children’s and general hospitals, military medical facilities, private otolaryngology and audiology practices, and schools. The uses include post-screening assessment, diagnostics, and threshold estimation.
Clear ABR results have been obtained in NICUs, ORs, and places that clinicians reported were “impossible to do ABR” before they tried the new system. ABR results were obtained from nonsedated newborns, infants, and young children, and in patients with sleep apnea, autism, cerebral palsy, and other conditions that clinicians know were difficult to test with ABR. Figure 3 shows ABR results recorded with the system from a number of patients—with very low noise and clear responses.
Clinical Importance and Benefits
Eliminating sedation is of paramount importance. Without sedation, many more patients can be candidates for EP tests, such as patients of audiologists who do not have access to a sedation facility, patients with medical conditions counterindicated to sedation, and patients who require multiple ABR tests. Another group is patients with such conditions as sleep apnea; sleep only increases muscle artifact and makes ABR results unattainable. Such patients may also include malingerers who would not consent to a sedated ABR test, but would deliberately flex their facial muscles (grind teeth) to obscure ABR results. Additionally, ABR tests can be administered more often, for example to monitor hearing status in infants or patients undergoing potentially ototoxic treatment.
Ability to operate in electromagnetically harsh environments, where strong EMI is present, such as operating rooms ORs, NICUs, ICUs, doctor’s offices, schools, etc, enables reliable ABR to be recorded without Faraday cages, thus making ABR practical for testing any patient in any clinical environment.
Economic benefits of the system are related to eliminating the need for sedation, postsedation care, and insurance premiums associated with sedation, as well as eliminating the need for Faraday cages.
Sedation costs. There are two sedation options being utilized today: The first option is sedation in the primary care physician’s or audiologist’s office, typically using chloral hydrate. The cost of this technique is approximately $265 and includes the cost of the physician and nurse or nurse practitioner’s time administering the drug. The second technique is a deep-sedation analgesia protocol estimated at $22.70 per minute.10 Assuming a 90-minute time slot, we estimate an average cost of sedation for ABR at $2,043 per occurrence. Failure to sedate sufficiently while accomplishing the procedure through restraint and failing to complete the task through inadequate sedation will lead to additional costs in the post-anesthetic care unit (PACU).
Post-sedation care costs. Oxygenation should be monitored until patients are not at risk of hypoxemia. If fentanyl is used as the analgesic agent, most patients are at minimal risk of hypoxemia within 60 minutes of the last dose. Other opioid analgesics may require longer periods of observation. A clinic that implemented the earlier AAP guidelines for pediatric sedation prospectively followed up 1,140 children who were sedated for procedures by nonanesthesiologists using a quality assurance tool; approximately 13% of the children received inadequate sedation. They also reported a 5.3% incidence of respiratory events, including one in which a child had apnea that required extensive resuscitation.11
A recognized concern of pediatric sedation is that these patients can easily “slip” from one level of sedation to another, regardless of the sedating drug used. If the patient encounters a complication during sedation (undersedated or oversedated), there are additional costs associated with sustained recovery, observation, and monitoring in the PACU: $2,540 with undersedation and $3,310 with oversedation.12

|
| “A New Method for the Collection of AEPs,” by Isaac Kurtz, MHSc, PEng, & Yuri Sokolov, PhD, March 2004 HR. |
Substantial cost savings would result from removing the need for sedation. There are many variables that would contribute to the cost savings, including the entire cost of sedation, the treatment of complications resulting from sedation, and litigation costs associated with adverse sedation outcomes. The number of patients who undergo sedated ABR is considerable: For example, some hospitals sedate almost all infants referred from newborn hearing screening (NHS) programs for post-screening assessment. There are 4 million births per year; typical referral rates from NHS are from 1% to 4%, and there are patients of other age groups who undergo sedated ABR. It would not be unreasonable to estimate that cost savings nationwide could approach $1 billion annually.
Patient Care Benefits
There is no way to provide safe pediatric sedation to all patients. Avoiding sedation and all of the associated risks effectively raises the standard of patient care. Patients who were not candidates for sedation, and thus for ABR, now have a means to be successfully tested with ABR and provided the care they need. Children need not be subjected to the anxiety associated with a sedation suite, operating room, or soundproof booth. They can be tested right where they are, even in the comforting arms of a parent or caregiver.
References
- Hall JW III. Handbook of Auditory Evoked Responses. Boston: Allyn and Bacon; 1992.
- Hood L. Clinical Applications of the Auditory Brainstem Response. San Diego: Singular Publishing Group; 1998.
- M, Kwong B. Auditory brainstem response: Differential diagnosis. In: Katz J, ed. Handbook of Clinical Audiology. 5th ed. New York: Lippincott Williams & Wilkins; 2002:274-297.
- Sininger Y, Cone-Wesson B. Threshold prediction using auditory brainstem response and steady-state evoked potentials. In: Katz J, ed. Handbook of Clinical Audiology. 5th ed. New York: Lippincott Williams & Wilkins; 2002:298-322.
- Hall JW III. New Handbook of Auditory Evoked Responses. Boston: Pearson Education; 2007.
- American Academy of Pediatrics, American Academy of Pediatric Dentistry, Work Group on Sedation. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics. 2006; 118:2587-2602.
- Li X, Sokolov Y, Kunov H. System and Method for Processing Low Signal-to-Noise Ratio Signals. US Patent 6,463,411. Issued October 8, 2002.
- Li X, Sokolov Yu, Kunov H. System and Method for Processing Low Signal-to-Noise Ratio Signals. US Patent 6,778,955. Issued August 17, 2004.
- Kurtz I, Steinman A. Kalman filtering in recording auditory evoked potentials. In: Abstracts of the 28th Midwinter Research Meeting, Association for Research in Otolaryngology. February 19-24, 2005; New Orleans. Abstract 219:79.
- Lalwani K, Braukman J, Fonareva I. Office-based minor surgical procedures using a pediatric sedation service model. In: Abstracts of the American Society of Anesthesiologists Meeting. October 16, 2006; Portland, Ore: Oregon Health and Science University.
- Gallagher SM. Incidence and nature of adverse events during pediatric sedation/anesthesia for procedures outside the operating room: Report from the Pediatric Sedation Research Consortium. Pediatrics. 2006; 118.
- Lang EV, Rosen MP. Cost analysis of adjunct hypnosis with sedation during outpatient interventional radiologic procedures. Radiology. 2002;222:375-382.





