Summary:
A new digital reconstruction study shows that middle-ear hearing loss becomes severe when fluid occupies about half of the ear cavity, offering a non-invasive marker for diagnosing and staging otitis media with effusion.
Key Takeaways:
- Finite element simulations revealed a critical threshold at ~50% fluid fill, after which energy absorbance plummets and hearing loss rapidly worsens.
- Wideband Acoustic Immittance (WAI) results—especially low, flattened EA curves below 20%—could be used to identify when intervention is needed.
- The approach may improve pediatric diagnosis and guide treatment decisions or hearing-aid design by predicting sound transmission changes caused by residual fluid.
Otitis media with effusion (OME) is a leading cause of conductive hearing loss, especially in children. The condition quietly builds as sterile fluid accumulates in the middle ear cavity, muffling sound by stiffening the eardrum and slowing the tiny bones that relay vibrations to the inner ear. While tools like otoscopy and audiometry can detect fluid, they often fall short in pinpointing severity. Wideband Acoustic Immittance (WAI) testing can probe deeper into middle-ear mechanics, but translating its measurements into precise fluid volume estimates remains difficult. Due to these limitations, there is a pressing need for research that links specific Middle-ear effusion (MEE) levels to measurable changes in sound transmission and energy absorbance (EA) rate.
A collaborative team from China University of Mining and Technology, Xuzhou Medical University, and The Third People’s Hospital of Dalian has brought the middle ear to life—virtually. In research published (DOI: 10.26599/JOTO.2025.9540027) in the Journal of Otology, they harnessed a validated finite element model to simulate how different amounts of fluid alter the ear’s ability to transmit sound. Their digital reconstructions uncovered a clear cutoff point—around half the cavity’s volume—beyond which hearing quality rapidly deteriorates. This insight offers clinicians a new, non-invasive way to assess and stage middle-ear fluid buildup.
The study began with an FE model of a healthy ear, refined to closely match experimental measures of vibration, impedance, and EA. Six MEE scenarios were simulated: 25%, 50%, 64%, 75%, 82%, and 100% cavity filling. At 25%, changes were barely detectable—umbo and stapes footplate (SFP) motion declined slightly, producing 1–3 dB of hearing loss and negligible EA shifts. Trouble started at 50%, when fluid submerged the umbo: hearing loss rose to ~9 dB and EA rates fell to about 20%. Beyond this, the decline was steep. At 64–82%, EA flattened to 5–10%, while hearing loss reached 16–30 dB. At full filling, the EA curve approached zero, indicating almost total reflection of incoming sound, and hearing loss peaked at 46.47 dB. The steepest EA drop consistently appeared near 2000 Hz—a key frequency for speech understanding. By mapping these patterns, the researchers identified a functional “danger zone” for middle-ear fluid accumulation.
“Our simulations show that the middle ear can cope surprisingly well until fluid fills about half its volume,” said lead author Wen Liu. “Beyond that point, the system’s ability to transmit sound collapses. This is not just an academic insight—it’s a clinical marker. If we can identify patients crossing this threshold through non-invasive EA testing, we can intervene before hearing loss becomes severe. For children, especially, this could mean protecting language development and school performance.”
These findings could transform how doctors interpret wideband acoustic immittance results, turning EA curves into a direct measure of fluid severity. Patients with near-normal EA can be monitored, while those with rates below 20%—and a flattened curve—can be flagged for intervention, such as fluid drainage. The approach is particularly promising for pediatric care, where invasive diagnostics are challenging.
Beyond diagnosis, the model could also inform hearing-aid design, predicting how residual fluid alters sound transmission. By translating virtual simulations into clinical decision tools, the study bridges engineering precision with patient-centered care.
Funding information
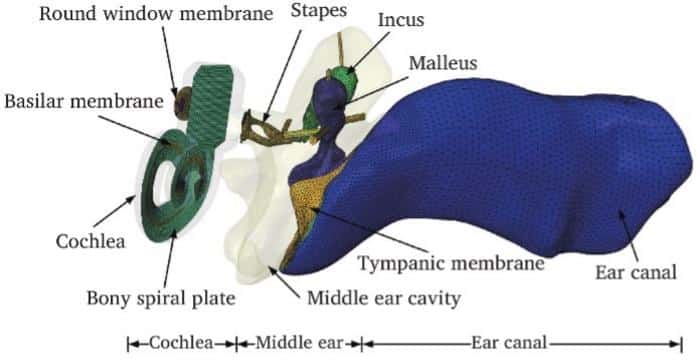
This study is supported by the National Natural Science Foundation of China (52275296), the Priority Academic Program Development of Jiangsu Higher Education Institutions.Featured image: FE model of the normal human ear. Photo: Journal of Otology, Tsinghua University Press