There are many factors in fitting asymmetrical losses with hearing aids. This article reviews three commonly overlooked areas that relate to audiometric test results, assessment of the length of time the patient has been without amplification, and knowledge of the patient’s mindset regarding their amplification choices.

|

|
| C. Mike Hall, AuD, is an audiologist who lives in Walnut Creek, Calif, is retired from private practice, and is now serving as a consultant in the hearing industry. Carl Croutch, AuD, is an audiologist at Kaiser Permanente Hearing Center in Daly City, Calif. | |
Over the years we have had numerous valid and successful formulas devised and proposed for use in calculating the amount of gain and maximum output that should be used for fitting various hearing losses. The evaluation and fitting of amplification of patients with asymmetrical hearing loss require a different mindset on the part of the clinician. With the introduction of digital circuitry, many asymmetrical losses that could not be fit other than with a CROS or BICROS device now can be fit with digital hearing aids binaurally. The purpose of this paper is to discuss some of the more important factors that need to be addressed in order to improve success in the fitting of asymmetrical losses.
Asymmetrical Hearing Losses: General Discussion
The clinical definition of an asymmetrical hearing loss is where there is a difference between ears of greater than 15 dB or a difference in speech understanding scores of 20% or greater.1
CROS and BICROS. In the 1960s, Harford and Barry2 proposed a hearing device that fed the sound from the worse to the better ear via a wire. If the better ear was essentially normal, it was proposed to use Contralateral-Routing-Of-Signal (CROS). If the better hearing ear was aidable, it was proposed to use a BICROS device in which both ears had microphones and amplifiers, even though the better ear had less gain than the worse ear. Many patients rejected this choice because of having to fit and remove the wiring from one ear to the other each time they removed it or put it on.
Wireless options. Several years later, Telex patented a wireless version of the CROS and BICROS. However, these devices had their drawbacks, because the antenna needed to transmit the signal required a rather large device. Additionally, the electrical signal being transmitted from the poorer ear to the better ear was almost in real time, while the signal being transmitted to the better ear was at a much faster rate than the acoustical signal. With the CROS or the BICROS, the patient is hearing sounds from both ears, therefore they perceive that they are hearing sounds from both sides.
The introduction of digital circuitry has resulted in clinicians having to reevaluate and reexamine the fitting of amplification on patients with asymmetrical hearing losses. As technology has evolved with digital circuitry, we have learned two important things:
- Today’s digital processing and wireless transmission have a much faster processing rate, which in turn means that the acoustic signal being processed can be altered and presented to the ear at much faster rates than previous aids.
- With digital circuitry, we find that the flexibility enables us to be able to manipulate parameters that had only previously been “pipe dreams.” For example, with analog circuitry, the frequency response might be able to reduce the low frequencies 6-12 dB per octave; a digital circuit can reduce them by as much as 24 dB.
Along with all of the other manipulations that the digital circuit is able to control, the clinician now has a device that could only have been dreamt of in years past.
Some caveats in asymmetrical losses. The audiogram is often the first clinical indicator of a possible acoustic neuroma. If a patient presents with a significant asymmetrical loss and there isn’t a reasonable explanation, an appropriate referral for a medical workup and clearance is required before proceeding. Some of the pathologies that can cause asymmetric hearing loss include: congenital loss, head trauma, acoustic trauma, viral neuronitis, or meningiomas.
One key issue needs to be emphasized at this time. Clinicians are taught in training to perform retrocochlear testing to rule out the possibility of retrocochlear lesions—usually referring to them as acoustic neuromas. Keep in mind that there are many types of retrocochlear lesions that are not acoustic neuromas, including meningiomas, multiple sclerosis, and trauma.
Sometimes clinicians feel incompetent because their diagnostic testing did not show any indications of a retrocochlear lesion; it’s important to remember that the patient may have a lesion that is not in the category of acoustic neuroma.

|
| FIGURE 2. Audiogram for patient who is not a candidate for conventional amplification in the worse ear. |
Three Important Factors in Fitting Asymmetrical Losses
The following represents a list of what the authors consider to be some of the most important, and often overlooked, issues that should be addressed when fitting asymmetrical hearing losses:
- Audiometric test results;
- Length of time that a patient has or has not worn amplification in their worse ear;
- Patient’s mind-set regarding their amplification choices.
Audiometric Test Results
In this article we are going to avoid going into detail on ancillary subjects not directly related to fitting asymmetrical hearing losses. One of the subjects on which we will not go into detail are the various methods used for calculating the amount of gain a particular patient needs for his hearing loss. For the sake of simplicity, Figure 1 uses the 1/2-gain rule as proposed by Lybarger3 in the 1940s. Other formulas have been proposed advocating other methods of calculating the amount of gain that is needed to fit amplification, including POGO, NAL, DSL, and NAL2, as explained in various resources.4,5 Some methods use less gain, and most of the newer methods take into account the type of device (BTE, ITE, ITC, or CIC) being fit. For better or worse, correspondence with a number of clinicians suggests that the usual choice of fitting formulas is the method they have had the most success with depending on the patient’s hearing loss.
The brain combines the information at different frequencies from the signal that is picked up from the listener’s two ears. The clinician also needs to take into account the patient’s most comfortable listening (MCL) and the patient’s uncomfortable loudness level (UCL).
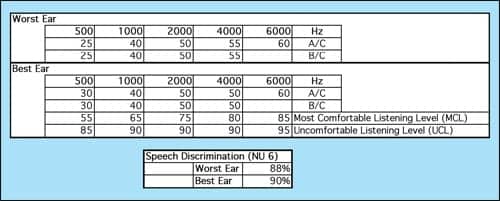
Figures 3 and 4 are examples of audiograms that illustrate which ears might or might not be candidates for a CROS or BICROS or where these two options might be the choice over conventional monaural or binaural devices.
In Figure 2, when the necessary gain is calculated, the worse ear is not aidable monaurally. Not only does the patient have a mixed hearing loss, but he also has tolerance problems, which reduces the dynamic range that is aidable. The speech discrimination in the worse ear is fairly poor. The poorer ear creates two dilemmas:
- The worse ear has a reduced dynamic range, which could partly explain the reduced speech discrimination score.
- The amount of power this patient’s worse ear requires makes the patient a poor candidate for amplification that feeds only into the poorer ear. Therefore, this patient would be a better candidate for a BICROS device or some other option.

|
| FIGURE 1. Lybarger 1/2-Gain Rule. |
Experience with Hearing Aids and Other Mitigating Factors
It has been observed that the longer a patient has not used amplification the more difficult it is for them to accept amplification. Many clinicians have debated the issue of the importance of auditory deprivation. Adult patients who had not worn amplification and had a long-standing loss were found to have more difficulty accepting amplification than patients who had lost their hearing recently. This observation greatly supports the “use it or lose it” argument. It does not mean you cannot fit patients with long-term unaided hearing losses; however, it does mean that they will probably have a more difficult time adjusting than patients who had been fit with a recent onset loss.
This also relates to the flexibility of the brain in adapting to changes that have occurred, whether from learning or lack of use. This phenomenon was dramatically demonstrated in patients who were fit with a cochlear implant. The brain has a great deal of neural plasticity.6 Whereas the implant’s initial signals will often be perceived as garble, with time and therapy, many patients find that they are able to enjoy the implant and function in a broad range of normal listening activities.
The brain, when provided with auditory stimuli, has a remarkable ability to adapt to changes as the result of damage or disease. If the brain is not stimulated, it will atrophy in the non-stimulated areas. This explains why patients who have not worn amplification for a long period of time find it difficult to adapt to amplification. An observation made by one of the early researchers of “neural plasticity” is that many of the patients who were fit with the original one-channel implant are still using them. With therapy, they eventually adapted to the implanted sound and their speech discrimination scores improved. This plasticity also helps us understand why some patients do not accept amplification for a previously unaided ear.
Other considerations. Another phenomenon that occurs on occasion is when a patient’s worse ear has cochlear damage that results in them having Tullio or Hennebert phenomenon. Clinically, you can validate this by perform a tympanogram. When the pressure change occurs, they will experience a Tullio phenomenon, or sound-induced vertigo, dizziness, nausea, and/or nystagmus.7 The same symptoms can be replicated with patients with the Hennebert phenomenon by having the patient suddenly exposed to a loud sound in excess of 100 dB HL.7
There are a host of other cochlear pathologies that can account for a patient with an asymmetrical hearing loss not being able to wear a hearing device in the worse ear. Some of these include the Mondini formation and Meniere’s disease. Both of these pathologies result in the cochlea not being able to process amplified sound when exposed to varying loudness. In addition, many of these patients have fluctuating thresholds that make it difficult for the clinician to set a hearing aid for one range of thresholds.
|
| FIGURE 3. Candidate for conventional binaural amplification. |
Counseling and Recommendations for Those with Asymmetrical Loss

|
|
Counseling patients with asymmetrical hearing loss requires a different approach, depending on the type of amplification choices they have. Patients with a normal ear and a dead opposite ear are a unique subgroup. These patients have a choice of using a CROS hearing device or a surgically implanted Bone Anchored Hearing Aid (BAHA), the latter of which has become an exceptional choice for single-sided deafness and conductive or mixed hearing losses.8 It should also be mentioned that patients with asymmetrical hearing losses have been shown to benefit from directional microphones and FM systems.
As mentioned earlier, how well a patient accepts a CROS or BAHA (or other appropriate recommendation) depends on how long they have had a unilateral loss. Patients with unilateral losses for many years are usually the most difficult to fit. They have had the “dead side” effect for so long that they have adapted to it. For example, if they go out to a restaurant, they habitually position themselves so their good ear is next to the person they are most interested in communicating with.
In our experience, a clinician usually gets only one try with these patients. If the hearing aid users don’t have the patience to condition themselves to wear a CROS aid, the result is usually a return for credit. Patients with a nonusable ear and a significant loss in the better ear are usually more receptive to amplification.
Discussion
The focus of this article has been to discuss what the authors view as commonly overlooked components in the fitting of patients with asymmetrical hearing losses. Sandlin and Bongiovanni9 provide an exceptional and in-depth discussion of this subject. They go so far as to say there are probably more than 60 criteria that should be considered in whether a patient is a candidate for binaural amplification versus CROS/BICROS amplification.
As mentioned earlier, the introduction of digital circuitry has provided engineers with greater flexibility in the design of newer amplification. Several manufacturers have offered wireless CROS/BICROS devices as dedicated products, and some of the new wireless hearing aids have features within their product lines that provide wireless CROS/BICROS benefits, widening the choices for our patients.
References
- Cueva R. Auditory brainstem response versus magnetic resonance imaging for the evaluation of asymmetric sensorineural hearing loss. Laryngoscope. 2004;114(10): 1686-92.
- Harford E, Barry J. A rehabilitative approach to the problem of unilateral hearing impairment: the contralateral routing of signals (CROS). J Speech Hear Disor. 1965;30:121-138.
- Dempsey J. Hearing aid fitting and evaluation. In: Katz J, ed. Handbook of Clinical Audiology. 4th ed. Baltimore: Williams & Wilkins; 727-728.
- Ross M. Hearing aid evaluation. In: Katz J, ed. Handbook of Clinical Audiology. 4th ed. Baltimore: Williams & Wilkins;1978.
- Dillon H. Hearing Aids. New York: Thieme; 2001.
- Doidge N. The Brain that Changes Itself: Stories of Personal Triumph from the Frontiers of Brain Science. Toronto: Penguin Group; 2007.
- Shaia W, Kartush J. Superior canal dehiscence. eMedicine from WebMD. Available at: .
- Flynn M. Overcoming conductive/mixed hearing losses and single-sided deafness with bone-anchored hearing devices. Hearing Review. 2007;14(9):44-48.
- Sandlin R, Bongiovanni R. Fitting binaural amplification to asymmetrical hearing loss. In: Valente M, ed. Strategies for Selecting and Verifying Hearing Aid Fittings. New York: Thieme Medical Publishers; 1994.
Correspondence can be addressed to [email protected] or C. Mike Hall, AuD, at .





