Five common clinical challenges and potential solutions to help solve ABR and ASSR dilemmas.
Neurophysiologic techniques such as auditory brainstem response (ABR) and auditory steady-state evoked responses (ASSR) are necessarily relied on to assess the peripheral auditory system and to estimate the hearing thresholds of infants and young children—primarily due to universal newborn screenings.
With an ever increasing emphasis on early detection and treatment of hearing loss, the importance of accurately estimating the audiogram via neurophysiology has increased. ABR evoked by brief tone-bursts and ASSR evoked by tonal stimuli both provide reliable frequency-specific information. However, the inherent small amplitude of these responses (at or near threshold) can make them difficult to detect and verify in even sleeping infants.



This article was submitted to HR by Todd B. Sauter, MA, instructor at University of Massachusetts Medical School, in Worcester, Mass; Douglas L. Beck, AuD, director of professional relations at Oticon Inc, Somerset, NJ; and David P. Speidel, MS, director of audiology services at Interacoustics, Eden Prairie, Minn. Correspondence can be addressed to Dr Beck at .
This article describes five common clinical challenges and potential solutions to help solve ABR and ASSR dilemmas (for more information, see Beck, Speidel, and Petrak1).
Challenge #1: Intermittent Myogenic Noisy Recordings
Intermittent myogenic noise may contaminate the ABR/ASSR response. Myogenic noise has a significantly greater relative amplitude than the ABR and ASSR—and myogenic noise spectrally overlaps the ABR and ASSR response—preventing analog and digital EEG filtering from completely removing the effects of the noise. Myogenic activity is significantly heightened in patients who are awake or moving. In the vast majority of ABR/ASSR evaluations in infants, sedation or anesthesia is not utilized, thus the ideal subject state is often not realized and myogenic activity may be a significant impediment.
Solution 1. Myogenic activity reduction is achieved by eliminating patient movement and is best accomplished through sleep. Therefore, verbal and written instructions to parents or caregivers regarding the importance of sleep for a successful test are of paramount importance.
Coordinating sleep or nap time to occur in accordance with the ABR/ASSR appointment is ideal. To increase the likelihood of a nap, breast- or bottle-feeding (in the test suite with appropriate privacy) immediately prior to testing is often useful.
Of note, myogenic activity can also be impacted by the physical position of the sleeping baby. Often, best test results are obtained when the child sleeps in their portable car seat, or sleeps while cradled on the lap of a parent or caregiver. A warm test environment with dim lighting also contributes to a sound sleeping state.
Solution 2. Signal averaging with Bayesian Weighting is a technology specifically implemented to combat intermittent myogenic noise. Typical ABR recordings treat all responses equally, which is only effective when noise conditions are ideally low. When myogenic and other noise is present, the artifact rejection level could be either lowered, which will cause a high rejection rate and prolong testing, or raised to accept responses with lower signal-to-noise ratios, thus degrading the quality of the recordings. Bayesian Weighting allows the use of a higher, more liberal rejection level without sacrificing data quality as each sweep is graded as “relatively” better or poorer and the better responses are weighed more heavily in the final response, thus ultimately stabilizing and improving the recording quality.
Challenges #2 & #3: Electrical Interference/Noise and Low-Amplitude Responses

Figure 1. Clinical confidence can be improved when evaluating the presence or absence of the response by viewing a graph of Fmp and residual noise.
Interference issues. Noisy recordings also may be attributed to ambient electrical interference. Some manufacturers offer a “loop back” device, which essentially bypasses the patient and turns your ABR equipment into an oscilloscope.
For example, Interacoustics offers the LBK-15. The LBK plugs into the transducer output jack and the recording electrodes connect to the device, as if it were hooked up to the patient. An integrated impedance check with a built-in resistance of 3000 Ohms verifies the integrity of the electrodes and specific protocols can be set up to evaluate the integrity of the test signal and to view the EEG display.
If a clear sinusoidal waveform is apparent in the data collection area, the hardware is producing the desired stimulus. If a “saw tooth” pattern is apparent in the EEG area, this typically indicates 60 cycle interference.
Low amplitude ABR/ASSR responses. Additionally, responses obtained at or near the physiologic threshold are, by definition, relatively low in amplitude, thereby challenging the waveform identification skills of the clinician and the automated detection algorithms within the software of ASSR devices.
Indeed, wave V of the ABR may decrease in amplitude to approximately 50 nanovolts or less as threshold is approached, requiring the clinician to employ all available techniques to enhance wave V amplitude and reduce noise.
Solution 1. The reduction/elimination of all noise sources is paramount. Physiologic noise (for example, myogenic noise, noted above), electromagnetic noise (from non-isolated alternating current, fluorescent lights, computers, large acoustic speakers, etc), and, of course, acoustic background noise in the soundfield/test area must be reduced or eliminated. Reducing and eliminating noise lowers the “noise floor,” (relatively) elevating the signal (with regard to signal-to-noise ratio [SNR]) and producing a “relatively” larger SNR with concomitant more easily identifiable and detectable responses.
Solution 2. Statistical analysis of multiple points also can facilitate the clinician in obtaining measurements. Modern online measures of SNR and residual noise are useful. Fmp (F = Statistical Analysis; mp = multiple points) and the Residual Noise Indicator are modern, useful tools to improve the accuracy and confidence in ABR and ASSR recordings. Fmp is a statistical analysis of the ABR recording that produces a percentage, based on the statistical confidence of the repeated detection of a response. The Interacoustics Fmp technology uses 21 time-locked measurement points for the response size (amplitude) and residual noise to provide a quantified confidence level. The repeated presence of the time-locked points is analyzed for each sweep, and less variation in the measures produces a higher confidence in the response.
Likewise, residual noise is calculated by measuring the differences in the noise values obtained from multiple points in each sweep. The amplitude of the noise is measured, and the lower the variation, the less noise in the tracing.
A graphical display of Fmp and residual noise is useful as it improves confidence with regard to evaluating the presence or absence of the response (Figure 1). It likely will reduce test time, and it relies on statistical data to interpret the neurophysiologic response, rather than the experience of the person conducting the test.
Solution 3. With regard to ABR, increased signal averaging also is useful when attempting to detect and identify wave V of the ABR. For adults, 2000 sweeps are often sufficient to achieve an adequate SNR for response interpretation in “neurodiagnostic” evaluations using high-intensity click stimuli. For infants, when using tonal stimuli at low sensation levels, signal averaging generally continues beyond 2000 sweeps. Unfortunately, extended signal averaging requires additional time, and, of course, as time increases, the opportunity for the child to wake up increases.
Therefore, an efficient stimulus rate must be utilized to reduce test time and increase speed of data collection. For clicks and high-frequency tonal stimuli, the full ABR waveform can be recorded within 15 milliseconds of the stimulus onset—even in infants and even when threshold is approached.2,3 A stimulus rate of 61/sec is useful and allows 1000 sweeps to occur in 16 seconds. Note that, for children with immature central auditory nervous systems (ie, children born premature) and for children with known neurological conditions, slower rates are utilized.
Solution 4. With regard to ASSR and ABR, the CE Chirp® stimuli solution impacts and reduces multiple challenges. For decades,4 it has been known that as the true threshold of hearing is approached (particularly with regard to the lower frequency spectrum), extremely long latencies (10 to 15 msec) and increasingly ambiguous waveform morphology occur. These negative features occur secondary to increased stimulus rise times and delayed cochlear travel times, negatively impacting neural synchrony.
Elberling et al5 acoustically engineered a stimulus (CE-Chirp) designed to overcome these problems, and it is especially beneficial for infant hearing screening and estimated hearing thresholds. The CE Chirp delivers lower frequencies first, with higher frequencies delivered later, thus providing a stimulus that takes into consideration cochlear travel time and uses it to maximize neural synchrony. This results in a more reliable and easier-to-detect response (for a detailed discussion of the CE-Chirp, see Beck et al6 and Cebulla et al7) that is often twice as large as the traditional stimuli, and generally requires less test time. Chirp stimuli have been developed in both broadband models, as well as narrow-band models, to be used when more frequency specificity is required. Data comparing narrow-band chirp with tone-burst ABR thresholds as predictors of audiometric thresholds is detailed in Don and Elberling.8
Challenge #4: Small Infant Ear Canals
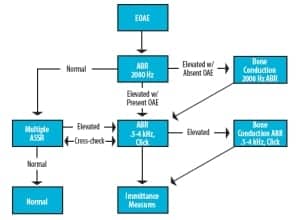
Figure 2. Example of an infant evaluation flowchart/protocol.
The physical size of the infant ear canal presents practical and acoustic challenges. Insert earphones are typically favored over supra-aural headphones for many reasons including:
- Increased inter-aural attenuation;
- Avoidance of ear-canal collapse;
- Patient comfort; and
- Minimization of stimulus artifact and diaphragm ringing.9
Typical foam ear-tips are often too large for placement in the neonate/infant’s tiny canal and smaller plastic tips can easily be dislodged.
Solution 1. One solution is to manually “debulk” the foam eartip, thus allowing a tighter “roll” with an improved chance of insertion success and effective retention. Further, it is important to consider the acoustic differences between the adult and infant ear canal when using insert earphones. The effective SPL value at the eardrum in infants will be significantly higher than in adults—especially at frequencies above 2000 Hz, where the difference can exceed 10 dB or more.10
Challenge #5: Gathering Complete Data in a Single Evaluation
The Joint Committee on Infant Hearing (JCIH) recommends all infants referred for diagnostic evaluation should be tested using frequency-specific ABR (with bone conduction when necessary), evoked otoacoustic emissions (OAEs), and tympanometry using a 1000 Hz probe-tone supplemented as necessary with click ABR, acoustic stapedial reflexes, and ASSR. Recent data indicated a well-prepared infant will sleep 49 minutes on average.11 Therefore, clinics should have well-developed protocols designed to prioritize the most important data-gathering at the onset of the evaluation.
Solution 1. The following is an example of one such clinical protocol (Figure 2) designed to efficiently gather data using the test-battery approach. Once a patient is satisfactorily quiet (ideally, soundly asleep), obtain OAEs to indicate grossly normal or abnormal outer-hair-cell function and to indicate whether bone conduction testing should be prioritized in the evaluation.
ABR/ASSR testing then proceeds with intensity levels at or near what is considered the maximum acceptable threshold level for a normal-hearing child. These levels may differ based on stimulus/recording parameters used and should be based on carefully researched published data,12 as well as local clinical norms in infants. (Author’s Note: At UMass Memorial Medical Center, normal ABR/ASSR thresholds have been determined to be 20 dB nHL at 2000 and 4000 Hz and 30 dB nHL at 500 and 1000 Hz. These ASSR levels are lower than some published data, likely due to differences in stimulus and analysis techniques using the Interacoustics ASSR system and other devices. Investigation remains ongoing.)
Others prefer to begin with a brief ABR recording using high-intensity (eg, 80 dB nHL) click stimuli in order to rapidly obtain a clear waveform that provides valuable latency data.9,13 Nonetheless, all authors appear to agree the vast majority of test time should be spent close to physiologic thresholds.
If air-conducted ABR/ASSR thresholds are elevated, bone-conduction testing should proceed immediately in order to determine the type of hearing loss. It should be noted that, while 1000 Hz tympanometry is capable of indicating middle ear dysfunction in infants, it is not reliable in determining with certainty the conductive or sensorineural nature of a given hearing loss or the size of an air-bone gap.
Conclusion
ABR and ASSR techniques and protocols allow the audiologist to ascertain important knowledge about the hearing status of newborns and others unable or unwilling to respond via traditional behavioral audiometry. Nonetheless, ABR and ASSR techniques and protocols change, develop, and improve over time, as do software and hardware—thus allowing a constant improvement in clinical protocols, resulting in increased accuracy with regard to hearing threshold estimation. This article has offered five common problems associated with ABR and ASSR and multiple potential solutions (for more information, see Beck et al1).
References
- Beck DL, Speidel DP, Petrak M. Auditory steady-state response (ASSR): a beginner’s guide. Hearing Review. 2007;14(12):34-37. Available at: www.hearingreview.com/issues/articles/2007-11_03.asp
- Picton TW. Human Auditory Evoked Potentials. Plural Publishing: San Diego; 2010.
- Sauter TB, Tudrick JM, Seymour NM. Stimulus rate for high-frequency tone-burst ABR in infants. Presented at: American Academy of Audiology annual convention; April 2008; Charlotte, NC.
- Davis H, Hirsch SK. A slow brainstem response for low frequency audiometry. Audiology. 1979;18:445-461.
- Elberling C, Don M, Cebulla M, Stürzebecher E. Auditory steady-state responses to chirp stimuli based on cochlear traveling wave delay. J Acoust Soc Am. 2007;122:2772-2785.
- Beck D, Speidel D, Craig J. Developments in auditory steady-state responses (ASSR). Hearing Review. 2009;16(8):20-27. Available at: www.hearingreview.com/issues/articles/2009-08_02.asp
- Cebulla M, Stürzebecher E, Elberling C, Berger T. New click-like stimuli for newborn hearing screening. J Am Acad Audiol. 2006;18:725-738.
- Don M, Elberling C. Use of quantitative measure of ABR peak amplitude and residual background noise in the decision to stop averaging. J Acoust Soc Am. 1996;99:491-499.
- Hall JW III, Swanepoel D. Objective Assessment of Hearing. San Diego: Plural Publishing; 2010.
- Voss SE, Herrmann BH. How does the sound pressure generated by circumaural, supra-aural, and insert earphones differ for adult and infant ears? Ear Hear. 2005;26:636-650.
- Janssen RM, Usher L, Stapells DR. The British Columbia’s Children’s Hospital tone-evoked ABR protocol: how long do infants sleep, and how much information can be obtained in one appointment? Ear Hear. 2010;31:722-724.
- Van Maanen A, Stapells DR. Normal multiple auditory steady-state response thresholds to air conducted stimuli in infants. J Am Acad Audiol. 2009;20:196-207.
- Hall JW III. New Handbook for Auditory Evoked Responses. Boston: Allyn & Bacon (Pearson Education); 2006.
Citation for this article:
Sauter B.T., Douglas L.B., Speidel P.D. ABR and ASSR: Challenges and Solutions, 2012 Hearing Review. 2012;19(06):20-25.




