Summary:
The FDA has approved Med-El cochlear implants for infants as young as 7 months, expanding pediatric indications and enabling earlier access to sound and speech development.
Key Takeaways:
- Med-El now offers the only FDA-approved cochlear implant option for children starting at seven months old.
- Clinical trial data show strong safety and effectiveness, with over 80% of children demonstrating clinical success within the first year.
- Expanded eligibility for older children aims to improve hearing, language, literacy, and overall quality of life for those who do not benefit enough from hearing aids.
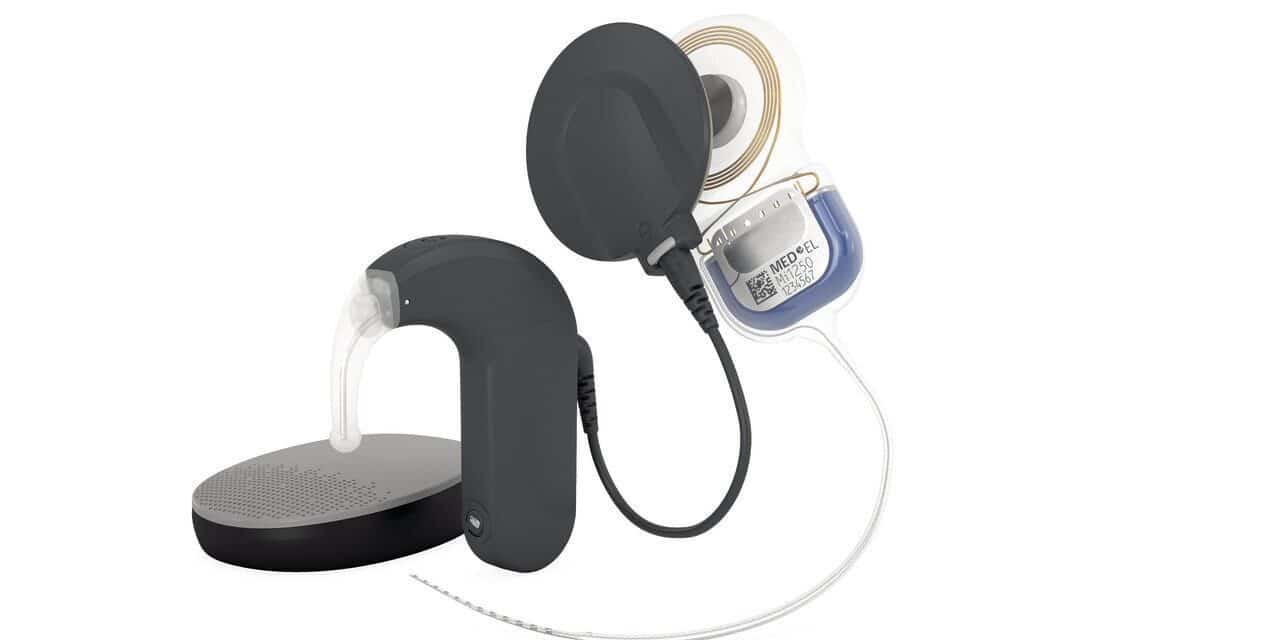
Med-El USA announced today that the U.S. Food and Drug Administration (FDA) has approved an expanded indication for Med-El cochlear implants for children seven months and older with bilateral sensorineural hearing loss (SNHL). This makes Med-El’s cochlear implant system the only FDA-approved option for infants this young, according to the company, offering the potential for earlier access to sound and speech development.
“Giving infants the opportunity to hear early in life is critical to maximizing the development of hearing and spoken language. It is extremely gratifying to have led this clinical trial demonstrating the safety and effectiveness of the latest implant technology for children as young as seven months. My hope is this will mean more children will experience the gift of sound soon after birth,” says Nancy M. Young, MD, Lillian S. Wells Professor of Pediatric Otolaryngology, medical director of Cochlear Implant Program, Ann & Robert H. Lurie Children’s Hospital of Chicago.
This FDA approval also includes expansion of audiologic and speech indications for children aged 12 months and older, reportedly offering the broadest pediatric indications of any hearing implant manufacturer.
“Many children with significant hearing loss use hearing aids but cannot hear all the sounds essential for understanding spoken language. They must work so much harder than their hearing peers. Expanding the eligibility of these children for cochlear implantation is so important to improving their hearing, language, literacy, and quality of life,” says Dr Young.
“This is an incredible step forward for families with young children with hearing loss,” says John Sparacio, president and CEO of Med-El USA. “Giving children access to sound as early as possible can make a world of difference for their future. We are committed to giving every child the best possible start in life through our closest to natural hearing philosophy paired with our advancements in safety and technology.”
Key Data Highlights:
● An FDA study in two groups of children shows that Med-El cochlear implants are safe and effective for children aged seven-71 months old with bilateral SNHL who cannot hear well enough with hearing aids.
● Overall, 110 of 123 children, including 81% in one group and 88% in the other group, showed clinical success with the cochlear implant in the first year.
● The rate of major complications was low in both study groups. All complications were known risks of cochlear implantation, and children implanted under 12 months old did not have more complications than other children.
● A post-approval study is planned to collect more data in children implanted at ages seven months to 17 years, 11 months who meet the new labeling criteria for Med-El cochlear implants.
For more information about Med-El cochlear implants, visit www.medel.com.