The House Research Institute (HRI) and Children’s Hospital in Los Angeles announced today that the United States Food and Drug Administration (FDA) has given final approval to begin a clinical trial of an Auditory Brainstem Implant (ABI) procedure for children. The trial is a surgical collaboration sponsored by the House Research Institute in partnership with Children’s Hospital Los Angeles and Vittorio Colletti, MD, of the University of Verona Hospital, Verona, Italy.
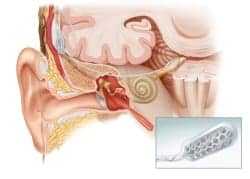
|
| Diagram of the Auditory Brain Implant (courtesy of House Research Institute). |
The ABI was developed at the House Research Institute and is the world’s first prosthetic hearing device to stimulate neurons directly at the human brainstem, bypassing the inner ear and hearing nerve entirely. Since the procedure began, more than 1,000 adults worldwide have received the ABI, according to House Clinic.
“This will be the first FDA-approved trial of its kind, and represents a major step forward to bring a sense of hearing to deaf children in the US who are born without a hearing nerve or cochlea (hearing organ) and therefore are unable to benefit from hearing aids or cochlear implants,” said Neil Segil, PhD, executive vice president for research, House Research Institute.
“Since its development at the House Research Institute in 1979 by Drs William House and William Hitselberger, the ABI has been successful in providing a sense of sound to many adults in the US. However, it has never been approved by the FDA for treating deafness in children. This study has the potential to expand the use of this device, which represents the only effective sensory prosthetic for direct brain stimulation in use today.”
The Pediatric ABI team includes physicians and researchers from the House Research Institute, including Eric Wilkinson, MD, Laurie Eisenberg, PhD, Robert Shannon, PhD, Marc Schwartz, MD, Laurel Fisher, PhD, Steve Otto, MA, and Margaret Winter, MS; Children’s Hospital’s Mark Krieger, MD, and Gordon McComb, MD; and Verona Hospital’s Vittorio Colletti, MD, Marco Carner, MD, and Liliana Colletti, PhD.
“We’re excited to have reached this milestone and look forward to being able to offer this amazing technology to children in the United States who currently have no other option for hearing rehabilitation,” said Eric Wilkinson, MD, co-principal investigator and lead physician for the clinical trial, HRI, and associate of House Clinic.
“Children’s Hospital Los Angeles is thrilled that the FDA has approved the Auditory Brainstem Implant clinical trial for children,” says pediatric neurosurgeon Mark Krieger, MD, chief of Medical Staff. Krieger also holds a position as chief of the hospital’s Division of Pediatric Neurosurgery. “We are looking forward to offering this innovative procedure to provide sound to deaf children in the United States. This prosthetic device has shown great success in providing hearing to children and adults and we look forward to contributing to research on advancing the ABI and to the training of physicians on the surgical implantation techniques.”
The clinical trial is part of a unique international consortium with the University of Verona for teaching and research to advance the use of the auditory brainstem implant in children worldwide. The ABI is already a successful treatment with the pediatric population in Italy, and the goal of the partnership is to bring the hearing implants to deaf children in the United States.
To date, children who have been implanted with ABIs outside the United States have demonstrated potential to understand speech, as well as to be mainstreamed in school. Currently, children in the United States who could benefit from an ABI must travel outside the country to have the surgery. Several of the children implanted in Italy work with the audiology team at HRI when they return to the United States.
“These children have never heard sound before, so their brains don’t know at first what to make of the neural signals coming in from the ABI,” said Robert V. Shannon, PhD, an investigator for the clinical trial, and director of HRI’s Koch Center for Hearing Restoration, who has been a leading scientist in the development of ABI device technology beginning with the first ABI for adults. “The pattern of information the ABI delivers to their brains is very different from the natural acoustic pattern. Yet their brain is eventually able to make sense of the information and many learn to speak and understand sounds. This demonstrates the amazing power of the brain to learn new patterns of information.”
The researchers anticipate securing final institutional approvals and funding commitments in the next several months, clearing the way for the study team to begin to evaluate patients for potential enrollment.
“The pediatric ABI clinical trial is yet another example of the House Research Institute’s leadership in developing new treatments for hearing loss and related disorders,” said James D. Boswell, CEO, House Research Institute. “We are grateful to the FDA and proud to be the sponsor of this clinical trial that will offer hope of hearing to children in the US who cannot benefit from hearing aids or cochlear implants.”
Source: House Research Institute




