HEI has since become one of the largest ear and hearing-related research centers in the world, and also serves as the site for many ongoing scientific investigations. The institute is dedicated to discovering the causes of ear diseases, hearing loss, vestibular disorders and developing treatments for all kinds of hearing problems. It also has taken a leading role in the creation of prosthetic devices designed to counter the effect of hearing loss, as well as find prosthetic and medical solutions for many unsolved causes of deafness.
One of the Institute’s most recent achievements is the development and U.S. Food and Drug Administration (FDA) approval of the Nucleus 24 Multichannel Auditory Brainstem Implant (or ABI). The device is for use by patients who suffer from Neurofibromatosis Type II (NF2). This condition usually manifests in early adulthood, when tumors grow on both the right and left auditory nerves and sometimes other places in the body. Removal of the tumors often necessitates the severing of the auditory nerve, causing total deafness. The ABI is designed to stimulate the region of the brainstem that normally collects the electronic signals from the ear.
The Institute gained its international reputation for the development of the single-channel cochlear implant, first implanted in the U.S. by Howard House’s brother, William F. House, MD, in 1960. Today, an estimated 25-30,000 people worldwide have received cochlear implants. According to John House, at least 80% of the patients who are implanted today have functional hearing capabilities to the level where they can use the telephone with their implant. Through extensive research, the Institute has found the causes of many types of hearing loss and can even prevent certain types of hearing loss. In many cases, hearing in patients is restored surgically or is improved through medical treatments
Currently, the Institute’s scientific efforts are expanding into research on the cellular and molecular levels. The belief is that an exploration of the cellular structures in the auditory system and their function offers the possibility of finding cures for some of the causes of deafness.
Institute scientists are exploring the auditory system from the ear canal through the inner ear to the brain itself. John House believes that it will be possible to reverse nerve damage through the regeneration of hair cells. “Through our cell molecular biology research, we are exploring ways to duplicate or re-grow the sensory cells,” says House. “When that happens there won’t be a need for some of the treatments used today, such as cochlear implant surgery. I would never have predicted some of the things I’ve witnessed during my 25 years in practice. Over the next 25 years there will be probably be just as many or more advancements.”
Ongoing Research
Currently, HEI is focusing on developmental biology on the cellular and molecular levels. Scientists are researching the identity of genetic, cellular and molecular mechanisms of the inner ear, and examining gene function, pattern formation, cell differentiation and cycle regulation. Their research may enable other scientists to develop cures, treatments and prevention that address many aspects of hearing loss.

Andrew Groves, PhD, chief scientist of Molecular Development, is working on two projects: the genetic control of growth and differentiation in bird embryos and the regeneration of hair cells. “I am interested in how genes sculpt the ear and how they make the right cells form in the right places in the ear,” Groves says. “It has been known now for perhaps 20 years that the same genes that can be used to ‘switch on’ the ears of birds, frogs or fish are similar to those found in mammals such as mice and humans. We want to understand how genes control growth and differentiation. This is important because many of the problems related to hearing loss appear to be hereditary. We are moving from gene discovery and identification to understanding gene function. In two years time, we will know the specific sequence of DNA of every gene in a human. It already has been done in some experimental studies such as in flies, worms and plants. What we need to do is find out how genetic material is used to build the ear. Understanding gene function will help in terms of diagnostics. For example, a newborn’s hearing abnormality may be able to be predicted through prenatal screening.”
FACTS ABOUT HEI
|
Most of the work in this area involves experimentation with eggs of birds. “The chick embryo is readily accessible to the experiment,” says Grove. “We can take an egg that has been laid, cut off the top of the shell and the embryo is there. We can see it, photograph it and do transplantation surgery at a very young age. We can then put the egg back in the incubator so it can continue to develop. That is something that can be extraordinarily hard to do with mammals such as mice because you have to operate on the mother. We think we are justified in using animal models because the bird ear, in many ways, is very similar to the human ear. It has the same basic structure, although birds cannot hear quite as well as we can. Moreover, birds are able to regenerate their sensory hair cells after damage–something that mammals are unable to do. We hope to gain clues as to how to regenerate sensory hair cells in mammals by studying the process in birds.”
Neil Segil, PhD, chief scientist of Cell Growth and Differentiation, is conducting research on the regeneration of sensory hair cells. Regeneration of these cells does not normally occur, and if successful, this research could be used in devising a cure for hearing loss. “In order to understand why hair cells don’t normally regenerate, we need to know much more about the biochemical machinery that governs cell division in the inner ear,” Segil says. “Our approach has been to try to understand what that machinery is composed of and how it is regulated during embryonic stages. In the embryo, the cells that form the inner ear have to divide many times. The correct development of the inner ear depends on the cessation of the cell division at a precise time; if they don’t stop dividing malformations occur.”
Segil and his team have identified a number of molecules that are important in controlling cell division in the inner ear. “These are families of molecules involved in regulating cell division in all of the cells in the body, but in the ear, one of these molecules, p27Kip1 (a cell cycle inhibitor), is particularly important. If we remove this one molecule from the mouse by mutation, the cells of the inner ear no longer stop dividing at the right time and the animals are deaf. However, although the timing is wrong, the cells nonetheless stop dividing. That means that there are yet other undiscovered molecules that help get these cells out of the cell division cycle.”
One of the lab’s current research efforts is to discover these other molecules. “From a clinical standpoint,” Segil says, “the hope is that, by understanding the cell division process in the embryo, we will also understand what keeps hair cells from regenerating in adults. In the future, it may be possible to regulate cell division, and thus regeneration potential, based on manipulating these molecules; but we are not there yet.”
Humans start with about 15,000 hair cells in each ear. Once lost, they will never grow back. However, in many other animals, hair cells can regenerate. If a bird is exposed to intense sound that damages the cochlea, it can hear again in three weeks. When a hair cell is destroyed in a bird, one of the supporting cells receives a signal to divide and it makes two cells. One of those becomes a support cell and one of them a hair cell. Thus, it does two things: it replaces the hair cell and it also replaces itself. By doing that, the bird gets back its hearing.
Segil and Groves are trying to answer the question, If this can happen in birds, can it happen in mammals? “We don’t know if the support cells in mammals have the same regenerative ability as birds’ cells have,” says Groves. “Cell division is very hard to control in the adult. But if we can identify the cells from which the support cells originated inside the embryo, then we may be able to see if these cells are still present in the adult.”
“If they are,” Segil continues, “then we can explore ways in which we can arouse or stimulate these cells to divide when normally they don’t.” This is the subject of a collaborative investigation by the two developmental biologists.
Research on the movement and interaction of cells during development has been the task of Andres Collazo, PhD, section chief scientist of Inner Ear Development at HEI. His research involves using vertebrates, primarily fish and frogs, whose inner ears are similar to human inner ears and express many of the same genes. “What we are really interested in is what sort of factors are involved in ‘patterning’ the ear,” Collazo says. “The ear is extremely complex. It has to be laid out perfectly during the course of development in relation to each of the different clusters of sensory cells that detect balance and hearing. Our hearing cells are laid out in a precise geometry in the ear and need to be wired up correctly in order to function normally. My lab’s research is to understand what genes are involved in that sort of patterning, and why this cluster of cells form here instead of there in the inner ear.
“What we are discovering is that some of the genes that are expressed in particular parts may be playing a role in deciding where these different structures form during the course of development,” says Collazo. “We manipulate the system by removing half of the ear with the unusual result that a mirror-symmetrical ear then forms. This leads us to believe that the placement of genes in the sensory placode is important to predict the position of balance and hearing structures.”
Collazo says they are finding that labeled cells are moving and mixing during development more than previously thought. “This finding is important for our search for candidate genes that may be involved in ear patterning. For a gene to be a good candidate for future studies, it has to be expressed in the right place at the right time. Much of the research is being conducted with the hope of eventually preventing insults and malformations in the inner ear.
“We hope that these types of approaches will give us the insight to better understand inner ear patterning,” says Collazo. “Certainly, we need to understand how to minimize the different insults that a fetus can be exposed to. It looks like many malformations can be explained by mutations in single genes. However, it is probably a lot more complicated than that for most types of hearing losses, and it could be anything from alcohol during pregnancy to a wide variety of other environmental factors. If we can find a way to minimize any trauma to the cell, and if we can find a gene that could strengthen the cells’ resistance to these traumas, this might be something that could be used in the future. But, right now, we have a lot of candidates and very little functional data.”
Federico Kalinec, PhD, chief of the section on Cell Structure and Function, is researching the mechanism that amplifies sounds in the inner ear (i.e., the “cochlear amplifier”). His study has great implications for preventing hearing loss, as well as several aspects of hearing instrument science. “The sounds you receive are amplified inside the inner ear by a special mechanism,” Kalinec says. “This is very important for the sensitivity and frequency discrimination of the human ear”. Damage of the cochlear amplifier, either by aging, noise, antibiotics, drug treatments or other environmental factors, is a common cause of sensorineural hearing loss afflicting millions of people around the world.
Kalinec’s research involves the mature sensory cells, the outer hair cells, of young adult and aging animals. Outer hair cells are special sensory-motor cells that have the ability to elongate, shorten and change shape when stimulated by the incoming sound, providing the mechanical effector to the “cochlear amplifier.” Kalinec is optimistic that, by understanding how these cells work and the manner in which they contribute to the mechanism of amplification, his research can lead to preventing some types of hearing loss such as those induced by acoustic trauma or aging. He has found that the motility mechanism involves special motor proteins in the plasma membrane of the outer hair cell. “We have evidence that the movement of the outer hair cells is tightly regulated by dynamic changes in the cell skeleton, and I hope that in the near future we will have precise information on the mechanism that regulates cochlear amplification,” Kalinec says.
The Many Facets of HEI
Beyond its research laboratories, the House Ear Institute hosts a number of facilities that offers clinical services, education and statewide UNHS program implementation.
House Ear Clinic: The House Ear Clinic, the otologic practice established by Dr. Howard House and his Associates in 1943, specializes in the diagnosis and treatment of ear diseases, hearing loss and disorders related to facial nerves, acoustic tumors and the balance system. The medical practice, which includes 10 neurotologists and 15 audiologists, sees about 6000 new patients and about 30,000 returning patients annually. Approximately 1500 major ear surgeries are performed at the clinic each year, ranging from the removal of acoustic tumors to the repair of eardrums, as well as tumor removal surgeries involving the bones of the head, neck and skull base. Neurosurgeons associated with the Clinic assist with the skull-based surgeries.
Children’s Auditory Research and Evaluation (CARE) Center: HEI’s CARE facility is a multi-disciplinary clinic for children with hearing disorders and also serves as a professional training and research facility. The center works closely with physicians of the House Ear Clinic to provide evaluation and treatment of hearing and communication disorders in children and young adults up to 21 years of age. Professional services include diagnostics, fitting of hearing instruments and cochlear implants and rehabilitation provided in the areas of audiology, speech-language pathology, psychology, otology and neurology for all types and levels of hearing disorders. Graduate student and clinical fellowship programs at HEI provide professional training for audiologists at several educational levels.

Diagram of an ear.
“Through the efforts of the CARE Center and the research of Yvonne Sininger, PhD, we have perfected the methods of testing hearing in infants,” says John House. “Last year, the State of California selected the House Ear Institute to implement its newborn hearing screening program throughout Southern California. This allows us to identify and prescribe appropriate amplification at a much younger age. This early intervention will significantly improve language development among infants and children with hearing loss.”
If, after a period of time, physicians find that hearing aids are not benefiting the child, they may suggest that the parents consider a cochlear implant, which is being implanted in children 12-18 months of age.
Accredited CME Program: Through the Institute’s Visiting Physician and Temporal Bone Dissection Course programs, ENT doctors from around the world can visit the Institute for three months to enhance their knowledge on ear diseases, hearing and balance disorders.
The course, established in 1970, is a surgical dissection course offered eight times a year, with 14 doctors enrolled in each course. “The doctors learn to perform the types of surgeries which we developed here,” says Education Director Antonio De la Cruz, MD. “In addition to practical laboratory experience, they also observe live surgery beamed to the auditorium where they have two-way audio communication with the surgeon.”
Resource Library: The library contains a comprehensive assortment of medical books and journals that address all aspects of the ear available to researchers and physicians. Access to more than 450 medical and scientific databases along with library collections is offered in a CD-ROM workstation.
Family Camp: Every year, the House Ear Institute holds a three-day family camp for families with hearing-impaired children. The camp focuses on family issues and strengthening communication channels. It is intended to improve the children’s interaction with positive role models to help them develop self-confidence and increase their awareness of peer identity and support. Workshops inform the parents on child-raising issues related to their hearing-impaired child and allow siblings of the hearing-impaired child to share their feelings and concerns.
Community Outreach programs are a vital part of the Institute’s services to families with hearing-impaired and deaf children. These events enable the families to exchange information on hearing aids and implants, cope with family tensions and emotional problems, and provide advice on how to raise their children to be successful.
Sound Partners: Sound Partners is a hearing conservation program that raises awareness of noise-induced hearing loss among professionals in the audio, electronic and recording industries. HEI partners with industry leaders, publishers and trade associations, providing hearing screenings, educational workshops and hearing conservation information at major trade shows.
Lipreading Workshops: Lipreading Workshops are nine-week courses offered three times a year to teach speech-reading strategies, coping techniques and assertiveness to people adapting to diminished hearing.
House Ear Institute is located on 2100 West 3rd Street, Los Angeles, CA 90057. For more information on the facility, scientists and their research, visit the HEI website: www.hei.org.
Zarpan Osmani is associate editor of The Hearing Review.
|
The Challenge of Hair Cell Regeneration By Andrew K. Groves, PhD, and Neil Segil, PhD The Problem
More than a decade ago, it was discovered that, unlike humans and other mammals, hair cell regeneration does occur in birds and other “lower vertebrates.” Since that time, many attempts to bring about regeneration in mammals have been tried without success. Recently, with the aid of a grant from the National Organization for Hearing Research, laboratories of the House Ear Institute have undertaken a new project to examine the underlying biology of hair cell generation. We hope that by understanding where hair cells come from in the embryo and how their progenitors are controlled at the molecular level, we will better understand the reasons for the lack of regeneration in humans and, ultimately, discover ways of curing hair cell loss. The Goal A second complementary strategy does not rely on the persistence of hair cell precursors in the adult Organ of Corti, but instead seeks to transplant hair cell precursors into the adult cochlea to affect hair cell replacement. Our first goal is to identify the cells in the embryo that give rise to the sensory hair cells of the inner ear. These are the same strategies being taken by scientists whose goals are to cure other neurological disorders such as Parkinson’s disease and spinal cord injuries. For instance, recent progress in stem cell biology has allowed the identification of neuronal precursors that may someday be used for regenerating nerves or transplantation to form new nerves. Unfortunately, we do not yet know the nature of the sensory hair cell precursor, so our current work is aimed at identifying the cells in the embryo that give rise to the sensory hair cells of the inner ear. The Approach
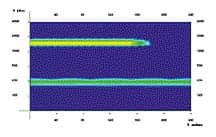
Our experiments have been made somewhat easier because of a new mouse that has been engineered in the laboratory of our collaborator, Dr. Jane Johnson at the Univ. of Texas, Southwestern. This mouse expresses a protein that glows green when fluorescent light is shined on it. Because of the way this mouse has been engineered, this Green Fluorescent Protein (GFP) is only expressed in newly born hair cells of the inner ear (see figure). So, if we want to track the origin of these cells, we need only watch for exactly when these cells start turning green and then look at which cells they are coming from. Of course, our ultimate goal is to purify the progenitors before they turn into hair cells. By purifying the progenitors we can study what makes them stop dividing permanently and perhaps how to make them divide again. It will also allow us to identify additional markers for hair cell progenitors that we can use to search for progenitors that may be present in the adult inner ear. Although it is believed that most of these embryonic progenitors disappear shortly after embryonic development, it is possible that some progenitors of hair cells persist in the adult inner ear, and that they are simply deficient in some way and so are unable to contribute to regeneration. The discovery of such cells would provide an important target for therapeutic attempts at inducing hair cell regeneration. This possibility is one of the motivating factors behind our quest for hair cell progenitors in the embryo. The Hope |