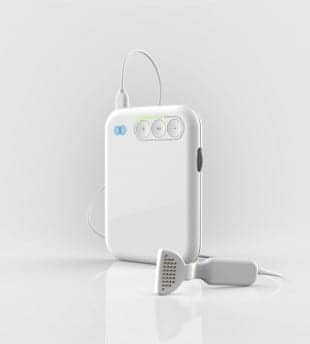
Neuromod Devices, a medical devices company in Ireland, has announced that it has secured the CE Mark—certified regulatory approval—for its mutebutton multisensory tinnitus treatment device. The announcement from Neuromod Devices states that tinnitus affects 10% of the UK population, according to the British Tinnitus Association, with 1% reporting significant secondary symptoms such as sleep deprivation, anxiety, and depression. At present there are limited avenues of treatment for subjective tinnitus sufferers and medical professionals alike.
“This certification is the culmination of 10 years of scientific and clinical research,” said Ross O’Neill, PhD, CEO of Neuromod Devices. “The medical device CE Mark is required for the approved sale of any medical device in Europe. It certifies the safety and efficacy of the mutebutton tinnitus treatment, and the competency of Neuromod Devices in being able to bring an approved medical device to market to treat this chronic condition.”
The mutebutton tinnitus treatment system, which was developed to address significant patient and clinical needs, reportedly combines synchronous audio and lingual (tongue) stimulation to promote patient neuroplasticity. According to Neuromod, when used for a minimum of 30 minutes a day over a 10-week period, the treatment has been shown to gradually reduce the sounds of tinnitus in clinical studies conducted by NUI Maynooth and the Hermitage Medical Centre Dublin.
“In the absence of clinically proven, readily available treatments, many tinnitus sufferers may have felt forced to try less effective treatments before now,” said Padraig Rushe, chief commercial officer of Neuromod Devices. “Many may have even tried lesser products or services, which are sold without the necessary regulatory approval. We are delighted to have secured our medical device CE Mark for the mutebutton tinnitus treatment.”
The mutebutton system launched in Ireland in December 2014. A wider European launch is scheduled for early 2015.
Source: Neuromod Devices