MED-EL USA announced that what is said to be “the country’s first” BONEBRIDGE Hearing Implant System surgery was completed at Texas Children’s Hospital. Otolaryngologist Yi-Chun Carol Liu, MD, performed the surgery on an 18-year old, and the device was successfully activated on November 13, 2018.
Cleared by the US Food and Drug Administration (FDA) in July 2018, BONEBRIDGE is said to represent “an entirely new approach to bone conduction hearing technology,” according to MED-EL’s announcement, and is cleared for use in conductive and mixed hearing losses as well as single-sided deafness for candidates age 12 and older. BONEBRIDGE is reportedly “the world’s first” bone- conduction implant that combines the benefits of intact skin with direct drive stimulation of the bone.
“Since the FDA clearance of BONEBRIDGE and ADHEAR earlier this year, we have been actively working with surgeons, audiologists, and hearing implant centers around the country to ensure that our game-changing surgical and non-surgical bone conduction hearing technologies can get to candidates,” said Raymond Gamble, CEO & President, MED-EL North America.
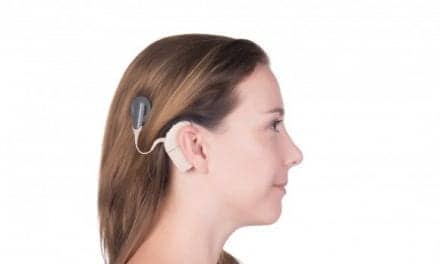
BONEBRIDGE consists of two components: the internal implant, and the SAMBA audio processor, which can be worn discretely beneath the hair. Unlike bone-anchored hearing aids with an abutment that protrudes through the skin and requires lifelong medical treatment and maintenance, BONEBRIDGE is said to offer “intact skin technology.” According to MED-EL, the implant is placed completely underneath the skin and the audio processor places minimal pressure on the skin. In a July press announcement from MED-EL, the company said that the SAMBA processor is equipped with adaptive directional microphones that help to identify and minimize noise interference in a setting like a restaurant. The SAMBA remote control allows users to choose from five different settings for television or music, among others.
Source: MED-EL
Image: MED-EL





I have single sided deafness from a scuba diving injury and am considering the bone bridge device. Anybody have personal experience or advice?
I would love to hear from people who have had the implant and can give me the pros and cons.
I am also interested in the bonebridge device. Any more reviews on this? Anyone one have bilateral bone bridge implants? I am a nurse and I am interested in whether this has bluetooth capabilities with a stethoscope.