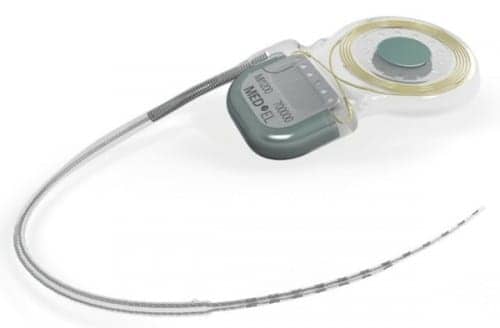
MED-EL USA, based in Durham, NC, has announced that it received FDA approval for the new Synchrony cochlear implant (CI), which can be used for MRI without magnet removal. According to MED-EL’s announcement, this approval means that the Synchrony cochlear implant, which will be available in spring 2015, does not require surgical removal of the internal magnet before a CI patient undergoes MRI.
“MED-EL has continued its unprecedented advancement in the area of cochlear implant and MRI safety,” said Raymond Gamble, president and CEO, MED-EL North America. “We are thrilled to be able to offer Synchrony here in the United States.”
MED-EL reports that Synchrony is compatible with all current MED-EL audio processors, including the recently approved Sonnet. Like all MED-EL implants, the Synchrony is designed to be ready to accommodate future technology as it becomes available. It is one of the smallest and lightest titanium cochlear implants on the market, the company says, making it a good choice for young CI candidates.
How New Magnet Design Changes MRI Scanning for CI Users
Magnetic resonance imaging (MRI) is a technique that uses a magnetic field and radio waves to create detailed images of the organs and tissues within the body. As previously reported in a November 26, 2014 article in The Hearing Review, cochlear implant users have historically risked magnet damage, displacement, and pain or discomfort during MRI. According to MED-EL, the Synchrony’s magnet freely rotates during MRI and self-aligns within its titanium housing, greatly reducing implant torque and the risk of demagnetization during MRI scans. This design enables high-resolution 3.0Tesla (T) scans without the need for magnet removal.
The conical shape of the removable magnet housing also reduces the risk of magnet dislocation or migration, reports MED-EL. The implant has a polymer stiffening ring within the silicone implant body to further secure the magnet housing. Additionally, the magnet can only be removed from the bottom side of the implant, making dislocation of the magnet due to trauma nearly impossible.
The implant is MR Conditional, allowing MRI scans at 3.0T with the magnet in place. The company reports that Synchrony’s magnet housing can also be substituted with a non-magnetic spacer for MRI head scans with minimal image distortion in the implant area. The removable magnet housing has a protective coating to prevent unwanted cellular adhesion, simplifying the removal and replacement of the implant magnet. Synchrony incorporates MED-EL’s Flex electrode arrays, which are engineered to preserve delicate cochlear structures for future advancements, and to provide complete coverage of the cochlea for strong hearing performance.
For more information about the company’s cochlear implant models and features, visit the MED-EL website.
Source: MED-EL USA