This weekend I watched Super 8, the J.J. Abrams a la Spielberg horror film that pits a group of teenagers against a spooky, massively powerful, unknown entity and the seemingly capricious rules of adults. Without spoiling it for you, the scariest part of the movie is when you know almost nothing about the “monster.” As with most horror films, once you actually see the “bogeyman,” he becomes much less terrifying
I think there are some elements of the above at play when talking about online hearing aid sales. For years, the hearing industry and professional organizations have struggled with what we often view as shadowy online marketers and their older siblings, mail-order and over-thecounter (OTC) hearing devices. These ersatz hearing aids come without the considerable benefits of professional care, a frightening proposition for most of us—and not just because it threatens our livelihoods. We understand the importance of individualized hearing aid fittings, counseling, and follow-up care (see my November 2011 editorial, “A Bit of Perspective Please“). But, for better or worse, the Internet does offer consumers a lot of information and options relative to amplification. And, as a practical matter, mail-order, wholesale, and drugstore hearing devices have always been fairly easy to ignore due to their small market share—7.6% of sales in 2008, a nominal increase (0.2 percentage points) over 2004, according to MarkeTrak VIII (October 2009 HR). In the past two decades, HR has covered many significant stories involving these devices, including:
- 1994: WhisperXL. Telebrands Corp offered the WhisperXL, a sub-$30 hearing aid that was mass-marketed in ads featuring comedian Steve Allen. Telebrands sold 400,000 units between April and November 1994 (roughly one-third of the total year’s sales of “legitimate” hearing aids), but eventually ran into legal problems (September 1996 HR). WhisperXL led many states to enact tougher regulations on mail-order devices.
- 2003: The FDA Citizen’s Petitions for OTC Hearing Aids. One petition advocated for the abolishment of the medical evaluation and waiver procedures prior to dispensing hearing aids, and the other petition requested a new hearing aid classification granting “over-the-counter sales, distribution and use status to one-size-fits-most hearing-aid-type devices that meet safety and efficacy requirements established by the rule.” For better or worse, the FDA denied both petitions in February 2004 (www.hearingreview.com/issues/articles/2004-03_10.asp). Most people cheered the decision. However, in my view, FDA has since adopted a policy of allowing anyone to market personal sound amplification products (PSAPs) with very little oversight (ie, don’t place the words “hearing” and “aid” next to each other, or, better yet, use the word “hearing device,” and don’t explicitly state that it compensates for a hearing loss, and—voila—your hearing aid becomes a PSAP).
- 2010: Boycottinternethearingaidsales.com. This Web site has attracted over 2,500 supporters since December 2010 (March 2011 HR online,). It recently changed its name to Baninternethearingaidsales.com (see this month’s Information Please for more details).
- 2011: UnitedHealth enters market. In October, hi HealthInnovations (HHI), a UnitedHealth Group (UH) subsidiary, announced it will offer discounted hearing devices to its members, as well as to consumers online.
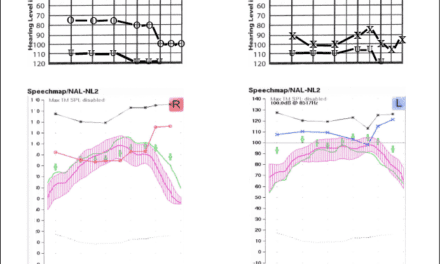
This month’s HR features an article (Methods for Prescribing Gain for hi HealthInnovations’ Hearing Devices: Reliability and Accuracy) by Dianne Van Tasell, PhD, a respected educator and researcher who has helped design the HHI clinical and home screening/prescribed gain systems. Although HHI repeatedly emphasizes its desire to build a robust referral network of hearing professionals, it’s difficult to objectively consider their systems without the idea that they bypass the traditional hearing care professional channel. The term “hearing aid” is also “conspicuous in its absence” in HHI’s materials.
So why is HR publishing this article? 1) It’s an important development, and we need to understand the underlying technology and rationale of the HHI systems so we can knowledgeably discuss them with patients and colleagues; 2) Industry researchers need to analyze it in a timely manner (ie, it will take several months for peer-reviewed journals to publish more detailed information); and 3) We need to stop fearing the online bogeyman, and that starts by understanding how these devices are used and distributed.
In my view, Dr Van Tasell’s article is exceptionally interesting and valuable, but it addresses only the technical aspects of hi HealthInnovations’ testing/fitting system. It does not address the critical issues of counseling, follow-up care, and aural rehabilitation. HR has invited Robert Margolis, PhD, an expert on automated audiometry (among many other things), to write about this topic in an upcoming edition. It’s our hope that these articles, and others to come, will help further the dialogue on this important issue.
Karl Strom,
Editor-In-Chief