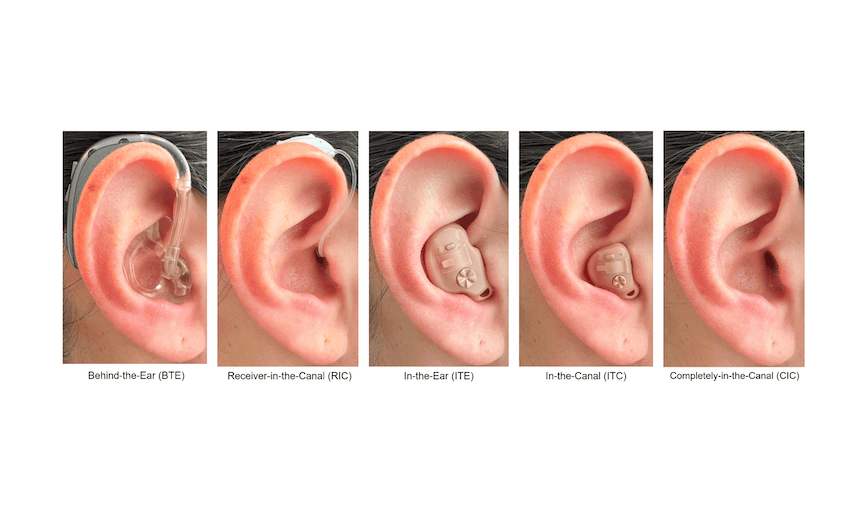
Featured image: Five styles of hearing aids, including behind-the-ear (BTE), receiver-in-canal (RIC), in-the-ear (ITE), and in-the-canal (ITC) devices. Photo: Eargo
By Lauren Pasquesi, AuD; Jackie Douda, MS; Jayaganesh Swaminathan, PhD
Having recently passed the one-year anniversary of enactment of the United States Food and Drug Administration (FDA) over-the-counter (OTC) hearing aid regulations, it is not yet clear how much progress has been made toward more widespread adoption of hearing aids or how OTC hearing aid users are responding to the new category of devices. To investigate the user experience, Eargo sent a survey to over 11,000 customers inquiring about their experience with Eargo’s OTC hearing aids. This survey provided insights into OTC hearing aid users’ experience, Eargo’s contribution to the changing landscape of hearing healthcare, and how a direct-to-consumer delivery and virtual support model like Eargo’s supports achievement of the intent of OTC regulations to improve access to hearing aids and lower costs.
Survey responses suggest that Eargo’s customer-first approach and innovative technology have improved time to adoption of hearing devices compared to established values in the literature, have positively impacted accessibility, contribute to perceived affordability and value, have helped to decrease the stigma of hearing aid use by offering a virtually invisible option, and have driven competition and innovation in hearing assistive technology, while continuing to validate the efficacy of OTC hearing devices.
Introduction
According to the National Institute on Deafness and Other Communication Disorders (NIDCD), approximately 28.8 million adults in the United States could benefit from the use of hearing aids. Although the need for hearing aids is high, usage continues to be low: 30% utilization for hearing aid candidates aged 70 and above and 16% utilization for hearing aid candidates aged 20 to 69.[1]
In 2017, Congress passed bipartisan legislation instructing the FDA to establish a category of over-the-counter (OTC) hearing aids. Congress was responding to leading medical and public health experts’ calls to improve access to hearing aids as a way to positively impact public health. For example, the National Academies of Sciences, Engineering, and Medicine (NASEM) identified hearing loss as a “public health and societal concern” and recommended the creation of a category of OTC hearing aids as a way to improve hearing care accessibility and offer a “wider range of options for adults with hearing loss.“[2] The President’s Council of Advisors on Science and Technology (PCAST) identified age-related hearing loss as a national problem and found that “untreated hearing loss is statistically associated with higher risks of social isolation, depression, dementia, falls with injury, and inability to work, travel, or be physically active.” PCAST also suggested the introduction of OTC hearing aids would rapidly increase innovation for and reduce costs of hearing aids.[3]
Consequently, the FDA issued a proposed rule in 2021[4] outlining their proposed regulations to establish separate categories for OTC hearing aids and prescription hearing aids.[5] Prior to the final OTC regulations, which were issued in August of 2022, air-conduction hearing aids were restricted devices, subject to certain conditions of sale, but were not prescription devices.
The FDA released the final rule (the Final Rule) after reviewing more than 1,000 public comments received during their rulemaking period.[6] Ultimately, when the regulations went into effect in October 2022, the FDA created two distinct categories for hearing aids: prescription and OTC; this framework replaced the restricted device framework that existed previously. As a result, previously restricted hearing aids were recategorized, with some marketed and sold as prescription devices and others as OTC devices.
There are broadly two kinds of OTC hearing aids: ones with preset amplification levels with limited customization capabilities and ones that are self-fitting. Self-fitting OTC hearing aids can be customized based on an individual’s hearing loss and require a higher level of review from the FDA compared to other kinds of air-conduction hearing aids, including most prescription air-conduction hearing aids. Most air-conduction and wireless air-conduction hearing aids, including those falling into the prescription category of hearing aids, are exempt from the FDA’s pre-market notification and clearance process; however, self-fitting OTC hearing aids require FDA 510(k) pre-market notification and clearance, a process that includes the submission of clinical data to the FDA, to validate the effectiveness of the self-fitting strategy. For ease of reference, in this article we refer to hearing aids purchased through a hearing care professional as “traditional hearing aids.”
In the Final Rule, the FDA states that hearing loss often results in a “significant impact on communication, social participation, and overall health and quality of life.” The FDA also references clinical literature demonstrating that the use of hearing aids has been associated with health benefits, improved social participation, and a better quality of life. Ultimately, the FDA opined that OTC hearing aids would spur more widespread adoption of hearing aids by removing barriers to hearing aid adoption, including, but not limited to, high cost, stigma of hearing loss and hearing aid use, perceived benefit relative to the price, convenience of purchase, and accessibility. The FDA also sets forth OTC hearing aid standards and requirements to ensure safety and effectiveness of these devices while fostering innovation to evolve hearing technology.
Eargo Inc. (Eargo) is a medical device company on a mission to improve hearing health. We believe that Eargo hearing aids are the first virtually invisible, rechargeable, completely-in-the-canal, FDA-cleared, self-fitting devices with virtual support indicated to compensate for perceived mild to moderate hearing loss. Our differentiated, consumer-first approach empowers consumers to take control of their hearing. Consumers can purchase online, at retail locations, or over the phone and get personalized and convenient support from hearing professionals via phone, text, email or video chat.
Methods
To understand the perception of individuals with hearing loss in the year since the OTC regulations became effective, Eargo surveyed customers who had purchased a set of Eargo OTC hearing aids directly from Eargo via the internet or phone between October 17, 2022 and May 31, 2023. Of the 884 respondents, 99.7% purchased hearing aids with self-fitting software. The questions asked in this survey were intended to aid in understanding whether Eargo’s direct-to-consumer business model, which includes virtual support (which we refer to in this article as a “direct-to-consumer service-delivery model”), was helping to improve hearing aid access, advancing the aims of the FDA’s OTC hearing aid regulations. Individuals did not receive any compensation for participating in the survey.
Results
Demographics
The average age of the respondents was 69 years, with 215 respondents under the age of 65. 75% of respondents were male, which aligns with estimates in the literature that, among adults aged 20-69, men are almost twice as likely as women to have hearing loss.[7]
The respondents were highly educated overall. The majority of respondents had a postgraduate or professional degree. Since our survey sample has attained more formal education than the populations reviewed in many hearing-related studies, this discrepancy should be considered when comparing our study against literature data.
The majority of respondents were retired (61%), with 37% employed full-time or part-time.
Respondents were analyzed cross-sectionally across all survey metrics. Basic demographics for the entire set and subgroups are noted below.

Like all OTC hearing aids, Eargo’s hearing aids are, in line with FDA regulations, appropriate for adults with perceived mild to moderate loss. The Final Rule enables customers to purchase OTC hearing aids “over the counter,” or, in other words, without a prescription or hearing test performed by a licensed healthcare provider. The Final Rule Requires that products are appropriately labeled to include the signs of mild to moderate hearing loss and that the labeling identifies the population for whom the hearing aids are intended. The vast majority of survey respondents (93%) indicated they perceived themselves to have mild to moderate hearing loss. Only three respondents responded that they had no hearing loss and 7% responded that their loss was more severe or profound. Because subjective perception of hearing loss can vary based on factors such as gender, age, race/ ethnicity, and education[8], this metric is more difficult to interpret.[9]
Experience with Hearing Care and Hearing Aids
We defined “Time to adoption” as the period between first recognition of hearing difficulty and purchase of a hearing device. Our respondents had an average time to adoption of approximately 4 years with approximately 80% adopting technology in 5 years or less and approximately 42% adopting within two years or less.
Approximately 70% of respondents were first-time hearing aid users. For respondents under 65 years of age and employed respondents, first-time hearing aid usage increased to 73% and 74%, respectively.
For respondents who had previously worn a traditional hearing aid, 80% had used their prior hearing aid for at least one year. Nearly half had used a traditional hearing aid for three years or longer.
Among respondents who had previously worn a traditional hearing aid, 75% reported that the performance of their Eargo hearing aid was equal to or better than their traditional hearing aid. 83% of those who had previously used a traditional hearing aid responded that using and maintaining their Eargo hearing aid was equal to or easier than using and maintaining their traditional hearing aid.
Respondents were also asked about the process of purchasing hearing aids from Eargo, and, if relevant, purchasing through the “traditional channel.” Of the subset of respondents who had previously purchased through the traditional channel, only 46% thought the process of acquiring a hearing aid through the traditional channel was quick and efficient, compared to 87% of that same subset noting the Eargo process was quick and efficient. Additionally, approximately 88% of all survey respondents (which include users who had only ever experienced Eargo’s process) indicated that the process of acquiring a hearing aid through Eargo was quick and efficient.
We specifically asked about barriers to purchasing hearing aids through the traditional channel. 76% of all survey respondents indicated that they believed there were barriers to purchasing hearing aids in-person via a clinic. The top three barriers were identified as 1) going in for multiple visits; 2) the types and styles of devices available in person; and 3) waiting for an appointment. These were consistent across all subgroups; however, respondents who are still employed rated taking time off work as a barrier equal to waiting to get an appointment (34% of respondents indicated each of these was a barrier).

Only 7% of respondents indicated they did not perceive any specific benefit to the direct-to-consumer service-delivery model that they had utilized in their purchase directly from Eargo. Most respondents identified that the biggest benefits from purchasing directly from Eargo were: 1) convenience; 2) transparent costs; 3) speed; 4) invisibility and rechargeability of the devices themselves; and 5) the technology of the virtual platform.

Interestingly, the majority of respondents had previously sought care in-person from a hearing care professional, despite ultimately purchasing from Eargo through its direct-to-consumer service-delivery model. Approximately 67% of all respondents (including those who had previously purchased hearing aids) had had a hearing test with a hearing care professional prior to purchasing Eargo, which was consistent with new users. This number rose to 76% in the population under 65 years of age.
Experience with Eargo Devices
58% of respondents reported that their Eargo hearing aids met or exceeded their expectations. While the responses to this question do not speak to any specific benefits received from the devices for any individual respondent, it is worth noting that users for whom Eargo was their first hearing aid were more likely to respond that the devices met or exceeded their expectations. It should also be noted that Kricos et al (1991) reported overall pre-fitting expectations of hearing aid performance were high among non-prior users of hearing aids.[10] 54% of respondents reported being satisfied or very satisfied with their experience with Eargo.
It is difficult to compare these two data points to the traditional channel, as clinic-by-clinic experiences vary and traditional hearing aid manufacturers and hearing care providers do not report on these metrics. However, based on our survey results that the majority of respondents who had previously worn traditional hearing aids found their Eargo devices to be as effective as or more effective than their prior hearing aids, we believe we can confidently draw the conclusion that Eargo devices and traditional devices may score similarly on questions of expectations and satisfaction.
Approximately 82% of respondents reported benefits from using their Eargo devices in four key listening situations: social situations/conversation, work and professional interactions, home life, or while using the phone.
Device Style Preferences
When shown pictures of five styles of hearing aids, including behind-the-ear (BTE), receiver-in-canal (RIC), in-the-ear (ITE), and in-the-canal (ITC) devices, survey respondents indicated a strong preference (87%) for completely-in-the-canal (CIC) devices, the same form factor as Eargo. Of all of the hearing aid choices, only 7% of respondents said they would prefer a RIC style device. When asked to select all the styles of devices they would be willing to wear, the majority chose only the CIC devices (58%). It should be noted that there is a selection bias in this question, as we surveyed current Eargo users who purchased a virtually invisible, CIC device, and we would expect that these individuals would have a strong preference for more invisible devices.

We asked additional questions about the importance of invisibility of hearing aids and found that 86% of users ranked invisibility as very important or moderately important when choosing a hearing aid. The number of respondents rating invisibility as very important increased significantly in the population under 65 years of age compared to all survey respondents (from 35% to 46%).
When asked if they still would have purchased a hearing aid if no invisible options were available, only half of survey respondents indicated with certainty that they would still purchase a hearing aid. This uncertainty increases significantly in the cohort of respondents that were first-time hearing aid users. Respondents were asked what they would have done if Eargo’s direct-to-consumer service-delivery model and its virtually invisible hearing aids did not exist. 8% of respondents said they wouldn’t have purchased any hearing device at all. 60% of respondents indicated that they would have sought in-person care but would have asked for an invisible device. Only 18% of respondents would have purchased a different, visible OTC hearing aid.
Discussion
The results of the survey indicate that OTC hearing aids, especially those with self-fitting features, are providing the benefits sought by the FDA in the Final Rule. The survey data suggest that OTC hearing aids are more acceptable to many respondents than traditional options, which we believe correlates with the significant reduction in time for adoption of hearing aids. Additionally, key results indicate that, based on the perceptions of survey respondents, Eargo hearing aids are seen as affordable, equal to or more effective than traditional hearing aids, are part of a more convenient delivery system, and are reducing concerns about stigma.
More Widespread Adoption of Hearing Aids
70% of survey respondents were new to hearing aids when they purchased Eargo. This indicates that Eargo is effectively reaching people with previously unaddressed hearing loss. Further, among survey respondents, the average time from when individuals recognized they had a hearing loss to getting their first hearing aid was approximately 4 years. This is much shorter than the literature estimate of 8.9 years.[11]

Accessibility
The majority of our survey respondents have seen a hearing care professional in-person for a hearing test, but at the same time, this group has not completed the process by obtaining hearing aids through the traditional channel. This reinforces the notion that there are perceived barriers, or at least drawbacks, to obtaining traditional hearing aids, even for those who start through the traditional channel. Our respondents identified the biggest benefits from using the Eargo direct-to-consumer service-delivery model to be convenience, costs, speed, and competitive technology, which mirrors the central tenets of OTC legislation. Responses from users also cite going in for multiple visits, the types and styles of hearing aids available, and wait time for an appointment as the primary drawbacks to the traditional model, which are factors that are mitigated when using the Eargo approach.
Effectiveness of OTC Hearing Aids
The majority of respondents who had previously worn traditional hearing aids found their Eargo devices to be as effective as or more effective than their prior hearing aids. Self-fitting hearing aids that are cleared by the FDA are held to a different regulatory standard than OTC hearing aids without self-fitting capability. To achieve FDA clearance, companies must have clinical data that demonstrates the effectiveness of their self-fitting strategy. Because Eargo’s current products, Eargo 5, Eargo 6, and Eargo 7, all use the FDA-cleared SoundMatch self-fitting software, it is unclear from our survey results if adults with hearing loss would find all OTC devices (in particular, those that are not cleared by the FDA as self-fitting) equally effective. However, it does affirm that the objective of the FDA’s regulations to provide a reasonable assurance of the safety and effectiveness of OTC hearing aids appears to have been achieved by certain FDA-cleared, self-fitting devices like Eargo’s.
Affordability and Value
Approximately 77% of respondents perceived that devices purchased directly online are more affordable or about the same cost as buying in-person, while only 3% of respondents perceived that devices purchased online are less affordable. A majority of respondents report benefit from using their Eargo devices in social situations, conversations, and their home life and that the devices met or exceeded their expectations. The perception that devices are more affordable, when combined with data indicating that OTC hearing aids meet expectations and are perceived to be as effective as traditional hearing aids, may help shift the perception of a lack of value of hearing technology as a barrier to adoption.
Stigma
Within the subset of respondents who were new users, only 40% indicated that they were certain they would still purchase a hearing aid if only visible device options were available. This indicates that an invisible product may actually be breaking a barrier to hearing care by increasing the likelihood of adoption. There was a strong preference of invisibility among all respondents, yet approximately 85% of the hearing aids sold today are BTE or RIC style devices.[12] While stigma is often discussed as a barrier to adoption of hearing technology, the broader availability of OTC devices following the FDA’s Final Rule and consumer behavior may provide clearer insights into the impact of stigma on hearing aid purchase and use.
Innovation
In response to a question regarding their expectations if Eargo’s service-delivery model and devices did not exist, 60% of respondents reported that they would seek in-person care but would ask for an invisible device. Only 18% of respondents indicated that they would have purchased a different, visible OTC hearing aid. This indicates that Eargo’s products, including the innovative technology within the virtually invisible form factor, is a driver for adoption of Eargo hearing aids rather than the mere availability of OTC hearing aids.
Transitioning to Eargo’s Direct-to-Consumer Service-Delivery Model
Evidence from this survey not only suggests benefits of the direct-to-consumer service-delivery model for new hearing aid users, but also for those who have been receiving ongoing care in the traditional channel. This subset of respondents, our “prior user” group, has invested significant resources (time, money, effort, etc.) over numerous years in the traditional hearing care pipeline, with nearly 80% of prior users utilizing traditional devices for 1 year or more and 46% utilizing for 3 years or more. These prior users have overcome the cognitive bias of the sunk cost fallacy, with survey data suggesting that their experience with the traditional channel was insufficient to prevent their personal shift to a new care model, in this case, Eargo’s model. This is even more interesting when we consider that the average lifespan of a hearing aid is approximately 5 years).[13] This suggests that their decision to shift from their devices purchased through the traditional channel to the OTC devices purchased through Eargo’s direct-to-consumer service-delivery model is even more impactful, as their traditional devices were most likely not used to their fullest longevity and use had ceased prematurely.
Conclusion
The work of NASEM and PCAST leading up to the regulations establishing a category of OTC hearing aids clearly identified hearing loss, especially untreated hearing loss, as a significant concern to public health and recommended several ways to lower barriers to hearing care and the adoption of hearing technology, including the introduction of OTC hearing aids. This survey demonstrates that certain OTC hearing aids and direct-to-consumer service-delivery strategies like Eargo’s are positively impacting accessibility, perception of affordability, stigma, and time to adoption. Notably, if only visible products were available, half of respondents indicated uncertainty whether they would obtain hearing devices, leaving their medical condition untreated. Continuing to encourage consumer-centric innovation in both technology and service-delivery models may deepen the impact of the OTC hearing aid regulations and help to minimize the prevalence of untreated hearing loss in years to come.
About the Authors:
| Lauren Pasquesi, AuD, holds a bachelor’s degree in Speech and Hearing Science from the Ohio State University and a Doctor of Audiology (AuD) degree from Northwestern University. After many years working clinically in an academic medical center and as an adjunct AuD program faculty, she now focuses on clinical audiology research. Lauren has experience in clinical care for patients of all ages, conducting qualitative and quantitative human-subject research, and acoustic and signal processing design. She is currently a senior research audiologist at Eargo. Contact: [email protected] |
| Jackie Douda, MS, is experienced in developing coding, coverage, and payment structures for disruptive technologies. As vice president of strategic reimbursement and access at Eargo, she works to advance patient access to innovative hearing technology through public policy development and via healthcare channels, including third-party payor coverage and traditional healthcare delivery models. |
| Jayaganesh Swaminathan, PhD, holds a bachelor’s and master’s degree in Electrical Engineering and a PhD in Speech, Language and Hearing Sciences. After obtaining his PhD from Purdue University, Ganesh completed postdoctoral training in the Research Laboratory of Electronics at MIT. Ganesh has experience conducting human perception research, developing speech enhancement algorithms in hearing devices, data analyses using statistical models and machine learning techniques, computational modeling, perceptual/acoustic evaluation of audio quality, signal processing, acoustics, and electroencephalography-based research in humans and animal models. He is currently the director of acoustics engineering and clinical research & development at Eargo. |
References:
- National Institute on Deafness and Other Communication Disorders (NIDCD). National Institute of Health (NIH). Quick Statistics About Hearing. March 2021. Accessed October 2023. https://www.nidcd.nih.gov/health/statistics/quick-statistics-hearing
- President’s Council of Advisors on Science and Technology. Report on Hearing Technology. October 2015. https://obamawhitehouse.archives.gov/sites/default/files/microsites/ostp/PCAST/pcast_hearing_tech_letterreport_final3.pdf
- Committee on Accessible and Affordable Hearing Care for Adults. National Academies of Sciences, Engineering, and Medicine (NASEM). Hearing Healthcare for Adults: Priorities for Improving Access and Affordability. 2016. Washington, DC: The National Academies Press. https://doi.org/10.17226/23446
- US Food & Drug Administration. Proposed Rule: Medical Devices; Ear, Nose, and Throat Devices; Establishing Over-the-Counter Hearing Aids. October 2021. https://www.federalregister.gov/documents/2021/10/20/2021-22473/medical-devices-ear-nose-and-throat-devices-establishing-over-the-counter-hearing-aids
- US Food & Drug Administration. Proposed Rule: Medical Devices; Ear, Nose, and Throat Devices; Establishing Over-the-Counter Hearing Aids. October 2021. https://www.federalregister.gov/documents/2021/10/20/2021-22473/medical-devices-ear-nose-and-throat-devices-establishing-over-the-counter-hearing-aids
- US Food & Drug Administration. Final Rule: Medical Devices; Ear, Nose, and Throat Devices; Establishing Over-the-Counter Hearing Aids. August 2022. https://www.federalregister.gov/documents/2022/08/17/2022-17230/medical-devices-ear-nose-and-throat-devices-establishing-over-the-counter-hearing-aids
- Hoffman HJ, Dobie RA, Losonczy KG, Themann CL, Flamme GA. Declining Prevalence of Hearing Loss in US Adults Aged 20 to 69 Years. JAMA Otolaryngol Head Neck Surg. 2017;143(3):274–285. doi:10.1001/jamaoto.2016.3527
- Kamil RJ, Genther DJ, Lin FR. Factors associated with the accuracy of subjective assessments of hearing impairment. Ear Hear. 2015 Jan;36(1):164-7. doi:10.1097/AUD.0000000000000075. PMID: 25158982; PMCID: PMC4272625.
- Eargo hearing aids are intended to amplify and transmit sound to the ear and thereby compensate for perceived mild to moderate hearing impairment in individuals 18 years of age or older. They are labeled and marketed for sale according to the FDA’s regulations. Survey respondents purchased these devices over the counter as permitted under the FDA’s regulations.
- Kricos PB, Lesner SA, Sandridge SA. Expectations of older adults regarding the use of hearing aids. J Am Acad Audiol. 1991 Jul;2(3):129-33. PMID: 1768880.Kricos, P. B., Lesner, S. A., & Sandridge, S. A. (1991). Expectations of older adults regarding the use of hearing aids. Journal of the American Academy of Audiology, 2(3), 129-133.
- Simpson AN, Matthews LJ, Cassarly C, Dubno JR. Time from hearing-aid candidacy to hearing-aid adoption: a longitudinal cohort study. Ear hear. 2019; 40(3):468-476. doi:10.1097/AUD..0000000000000641.
- Strom KE. Trends in hearing aid styles. Hearing Review. 2021;28(7):6,31.https://hearingreview.com/hearing-products/hearing-aids/trends-in-hearing-aid-styles
- Johns Hopkins School of Medicine. Frequently Asked Questions About Hearing Aids. 2023. https://www.hopkinsmedicine.org/health/treatment-tests-and-therapies/frequently-asked-hearing-aid-questions





It’s encouraging to see data on the effectiveness of the Eargo devices, and to learn they can be affordable. But decisive for me is how well an HA handles MUSIC. Not one mention of the lovely health-promoting stuff! And, as ever, clinical charts presented go no lower than 250 Hz. Small wonder the average user’s comment is typically “It sounds tinny”. C’mon clinicians, get talking with studio engineers, musicians, audiophiles. You’ll find they do speak the same language!