Tech Topic | January 2023 Hearing Review
The hearing healthcare provider, is uniquely suited to screen their patient’s dementia risk as it relates to their information and auditory processing abilities, audiometric findings, and ability to hear and listen in complex listening environments.
By Keith N. Darrow, PhD, CCC-A
Introduction
Hearing healthcare providers (HHCPs) must hold the welfare of each patient paramount by incorporating evidence-based clinical judgement.1,2 With more than 50 years of data3 indicating decreased cognitive function associated with the presence of hearing loss, the HHCP has a responsibility to understand, incorporate, and provide services to assess the adult patient’s risk of cognitive decline, including promoting the treatment of hearing loss which often positively impacts cognitive health4,5 and may reduce the risk of dementia.6
The notion that hearing loss impacts cognitive function was first documented when deficits in memory recall were observed at higher rates in persons with hearing loss.3 Nearly twenty years later, the increased prevalence of dementia was noticed in persons with hearing loss.7,8 For a thorough review of the literature on hearing and cognition, see Beck and Clark,9 Powell et al.10 and Beck.11
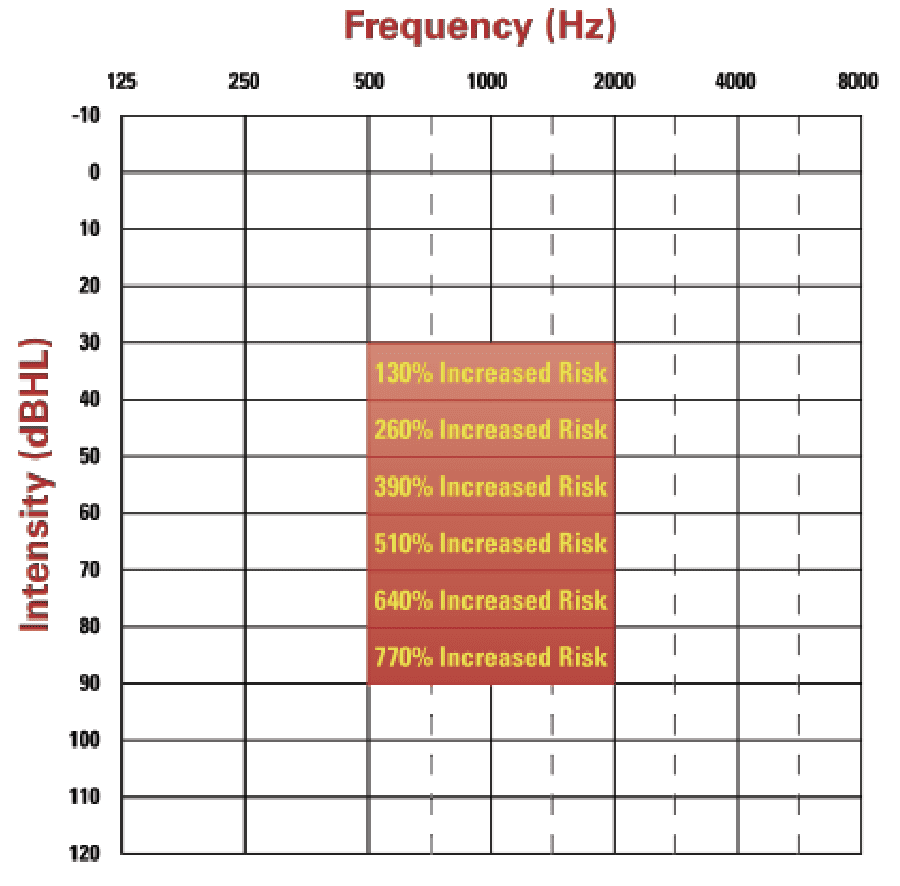
The shift to integrate cognitive health into hearing healthcare was propelled by Lin and colleagues who demonstrated the correlation of increased risk of cognitive decline and dementia with hearing loss, ie, the risk of cognitive decline and dementia increased an additional 130% per 10 dB drop in hearing (see Figure 1).12-14 Additionally, the emerging relationship of speech-in-noise handicap with incident rates of dementia15 have compelled many HHCPs to begin screening for cognitive decline (see the accompanying article by Weinstein, 2023, this edition of Hearing Review).
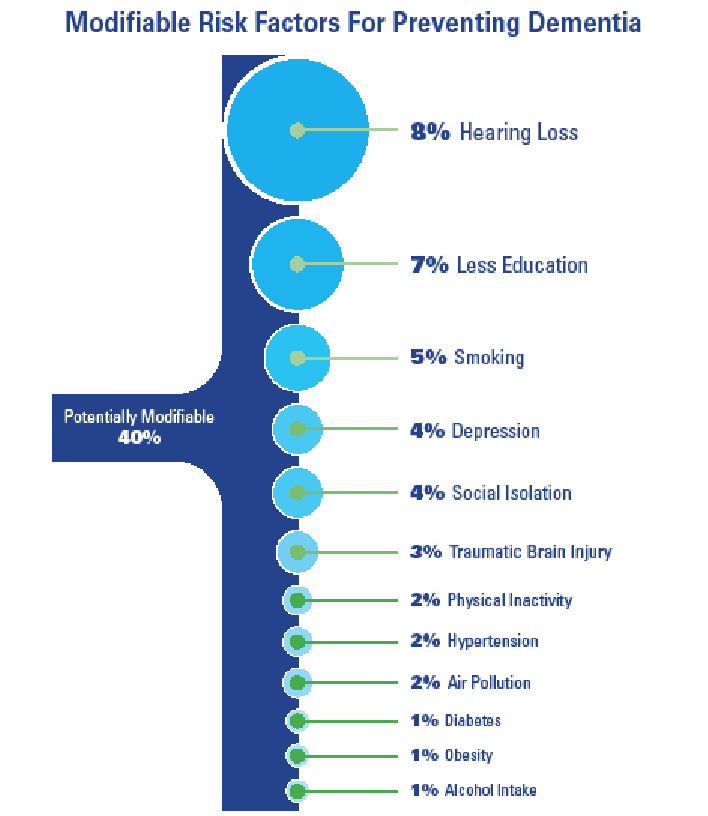
With 40% of all dementias considered potentially modifiable and 8.2% of all global dementia rates attributed to hearing loss,16,17 the HHCP is uniquely positioned to potentially reduce the patient’s risk of cognitive decline, or slow the progression of decline, thereby reducing conversion rates from Mild Cognitive Impairment (MCI) to Major Neurocognitive Disorder, e.g. dementia.
Understanding neurocognitive disorders as well as the age/genetic risks, the patient profile of the person with dementia (PWD), and the 12 modifiable lifestyle/health risk factors17 may all prove beneficial for the HHCP to increase their professional services and to actively refer appropriate patients for diagnosis and management.
Mild and Major Neurocognitive Disorders
The diagnostic classification for a person with diminished cognitive function has been revised to either mild neurocognitive disorder (MiNCD) or major neurocognitive disorders (MaNCD) formerly known as Mild Cognitive Impairment (MCI) and dementia, respectively.18 These newer terms clarify the presentation of the disorders by not including psychological illness and they reduce the stigma associated with the word dementia (i.e., dementia is derived from the Latin word meaning ‘insane’). Each of these disorders goes beyond the typical deficits of aging, which often include slower recall, limited issues with concentration, and maintenance of the normal capacity to learn. Mild Neurocognitive Disorder (MiNCD) describes an individual whose cognitive deficits do not interfere with capacity for independence in everyday living and typically entail a mild, or modest decline from a previous level of performance. In contrast, Major Neurocognitive Disorder (MaNCD) does interfere with the ability to perform daily independent tasks, also known as activities of daily living (ADLs). The person with MiNCD is at risk for developing MaNCD, with approximately 20% of patients converting to MaNCD on an annual basis.19
Cognitive deficits associated with neurocognitive disorders typically affect at least 1 (in MiNCD) or 2 (in MaNCD) cognitive domains, including complex attention, executive function, learning and memory, language, perceptual – motor/visual, and social cognition.
- Complex Attention: Generally involves sustained attention, divided attention, selective attention, and information processing speed.
- Executive Function: Generally involves planning, decision-making, working memory, responding to feedback, error correction, overriding habits, and mental flexibility.
- Learning and Memory: Generally involves immediate short-term memory, working memory, recent memory (free recall, cued recall, and recognition memory), and long-term memory.
- Language: Generally involves expressive language (naming, fluency, grammar, and syntax) and receptive language.
- Perceptual (Motor/Visual): Generally involves the coordination of visual and motor functions for everyday tasks such as picking up the telephone, handwriting, and using a fork/spoon/knife.
- Social Cognition: Generally involves the ability to interact with others.
Although hearing abilities are not explicitly mentioned in any of the cognitive domains, the ability to hear (perceive sound) and listen (ie, comprehend sound) are directly associated with complex attention (ie, the ability to follow a conversation with visual or auditory distractions in the background),20 executive functions (ie, the ability to perceive what others are saying and appropriately respond),21 learning and memory (ie, the ability to instantly associate ongoing speech with auditory memory to determine meaning and intent),22 language (ie, receptive language impairments resulting from hearing loss and other etiologies)23 and social cognition (ie, the known correlations of hearing loss and social isolation in older adults).24
The Person With Dementia
In addition to the previously noted inability to competently perform ADLs, the signs and symptoms of the PWD (MaNCD) vary and may include memory loss, poor judgment, difficulty communicating with others, wandering, repeatedly asking questions, anomia, loss of interest, and difficulty completing everyday tasks.25 These symptoms present gradually and represent a change from past behaviors. The HHCP provider must be acutely aware of these presentations and explore these behavioral changes during routine and thorough case history and clinical evaluations. It is important that these behavioral changes be reported to the primary care physician for diagnosis and management and to rule out pseudo-dementia or delirium, or pharmaceutical-induced causes, which may manifest as more typical MiNCD or MaNCD.
Clinical manifestation of the PWD, pseudo-dementia, and delirium can be similar; however, their etiology and treatment are considerably different. Pseudo-dementia, or potentially reversible dementia, is often the result of adverse drug interactions, emotional disorders (eg, schizophrenia), sensory loss (e.g., visual or hearing impairment), infection (e.g., urinary tract infection), nutritional deficits (eg, vitamin B12 deficiency), metabolic changes (eg, thyroid disease) or intracranial pathology (eg, head trauma or tumor).26 Delirium, on the other hand, is a rapid (ie, days to weeks) onset change in mental state and awareness resulting from acute brain dysfunction. A person with MaNCD can exhibit episodes of delirium, and these observations must be addressed immediately if suspected.27
The Prevalence of MaNCDs
MaNCD’s are a collection of heterogenous progressive neurodegenerative diseases that directly impact cognition and share a common feature of cerebral atrophy. The four most common types of dementia, include Alzheimer’s disease (AD), Vascular Dementia (VaD), Lewy Body Dementia (LBD), and Frontotemporal Lobar Degeneration (FTLD).
AD, the most common form of dementia, accounts for nearly 2/3 of all diagnosed MaNCD cases. There continues to be a lack of consensus regarding specific diagnostic criteria for AD, with most clinicians using the terms ‘probable’ and ‘possible’ AD.28 Typical AD diagnostic criteria include ruling out the presence of any clinical or other evidence of co-existing cerebrovascular disorders, medication-induced dementia (or delirium), any other condition affecting cognition, or any other known form of dementia.29 Indeed, all post-mortem cases of Alzheimer’s disease present with aggregate beta-amyloid plaques in the brain; however, these plaques are also found in individuals without diagnosed cognitive disorders. With the recent introduction of aducanumab as an approved treatment for mild AD, there is the potential for reducing the presence of amyloid plaques and potentially slowing the progression of the disease in selected candidates who are as of yet not clearly identified. Unfortunately, this targeted therapy does present with significant side effects.30
VaD accounts for nearly 20% of all cases of dementia. Vascular damage is often characterized by cerebral infarct, white matter lesions, myelin loss, and amyloid angiopathy, resulting in neuronal loss and synaptic degeneration.31 In addition to deficits in at least two cognitive domains, VaD diagnosis requires neuroimaging evidence of cerebrovascular disease.32,33 Further, type-2 diabetes is a significant risk factor for all types of dementia. Rates of VaD more than double in individuals with type-2 diabetes as compared to other forms of dementias. Although no targeted therapeutics exist for VaD, addressing cardiovascular health (e.g., lowering blood pressure, reducing cholesterol and anticoagulants, and controlling blood sugar) may slow the rate and potentially prevent further decline.3
LBD, like AD, is associated with abnormal protein deposits in the brain that can only be definitively diagnosed post-mortem. LBD is often associated with Parkinson’s disease (PD) and represents approximately 15% of all dementias. Diagnosis is often identified as concurrent cognitive impairment with supporting neuroimaging of the basal ganglia and REM sleep behavior disorder.35 LBD is the result of genetic mutations to the SCNA gene, identified as producing alpha-synuclein protein clusters (proteins normally found in the pre-synaptic terminal of neurons). These abnormal clusters of the alpha-synuclein protein are present primarily in the basal ganglia region in patients with Parkinson’s disease (PD) and are even more widespread in the brains of people with LBD + PD. The comorbidity of dementia in patients with PD can exceed 80% with a long enough survival time (i.e., > 20 years), implying dementia may be inevitable in PD.35-37 There is no targeted therapy for LBD other than those medications typically prescribed to patients with cognitive impairment (ie, cholinesterase inhibitors). Unfortunately, treatment targeting PD may only aggregate the LBD by increasing delusions, confusion, and hallucinations.
Frontotemporal Dementia, also known as FTLD or Picks Disease, is considered the earliest-onset neurodegenerative dementia38 with an average age of diagnosis of 53 years.39 This MaNCD accounts for approximately 5% of all dementia cases.40 As the name implies, primary lesion sites in the brain include both the frontal and temporal lobes, causing significant personality and behavioral changes, motor neuron disease, and accompanying non-fluent aphasias.39 There is no specific treatment for FTLD. Interestingly, the most common medications often prescribed for AD to temporarily relieve cognitive symptoms may exacerbate the symptoms of FTLD. Most often, antidepressants, antipsychotics, and speech-language therapy are indicated to manage the symptoms.
Pharmaceutical Treatments for MaNCD
With approximately 60% of all dementias considered the result of genetic predisposition (ie, the presence of APOE genes and first-degree family history) and the other 40% considered modifiable and potentially preventable,17 there must be a significant emphasis placed on both treatment and prevention. Currently, there are no widely available pharmacological interventions to directly target the cause of MaNCD, regardless of type. Most prescribed medications for MaNCD intend to moderate the symptoms of cognitive impairment, including memory and learning deficits, behavioral changes, and depression.
Acetylcholinesterase inhibitors (eg, Aricept, Razadyne, and Exelon). Acetylcholine (AcH) is a primary neurotransmitter of the central nervous system (CNS) and essential for processing memory and learning. Levels of AcH are typically reduced in the CNS of patients with MaNCD. Therefore, acetylcholinesterase inhibitors, which increase the amount of free AcH in the synapse, are often prescribed to patients with mild to moderate AD. These medications, administered orally or by patch, may improve or stabilize the symptoms of dementia for up to 1 year.41
Glutamate antagonist (eg, Namenda). Glutamate, another primary neurotransmitter of the CNS, is involved in learning and memory and is critical for neuronal survival. Unfortunately, excessive amounts of glutamate promotes cell death and may be an underlying cause of neurodegeneration in MaNCD. Glutamate antagonist may be prescribed in moderate to severe dementia to limit the damage caused by the excessive release of glutamate.
Antipsychotics (eg, Risperdal, Abilify, Zyprexa, etc.). This class of drugs may offer modest effects in treating psychosis, aggression, agitation, and erratic behaviors more common in late-stage dementia.42,43
Antidepressants (eg, Celexa, Zoloft, Prozac, Paxil, etc.) Depression may be difficult to diagnose in patients with MaNCD and may be a prodrome to the disorder. The two disorders may also share a common etiologic pathway, particularly white matter disease.44 When depression is present in MaNCD it can further reduce the quality of life, increase disability, and reduce the lifespan.44,45 Many patients with dementia are prescribed antidepressants, although evidence of efficacy (as defined by depression rating scales) is moderate at best.
Aducanumab (eg, Aduhelm). The FDA recently (July 2021) approved aducanumab to treat the underlying pathophysiology of AD and is recommended to treat MCI and mild AD.46 This human monoclonal antibody specifically targets the buildup of amyloid plaques in the brain. Initial studies found slowing of clinical decline as measured by the Clinical Dementia Rating and Mini-Mental State Examination scores.47 However, additional studies did not provide evidence of improvements in cognitive function. Ultimately, aducanumab was fast-track approved by the FDA based on its ability to reduce the presence of amyloid plaques without any corroborating evidence of less cognitive or functional decline.48 The required FDA confirmation study is anticipated to be complete in 2026. The side effects of aducanumab include a 40% incidence of brain swelling and bleeding. Aducanumab has been rejected by the European Medicines Agency.
Preventing MaNCD
Approximately 55-60 million people globally are affected by dementia, with rates anticipated to nearly triple by 2050.49 This increase is due to a substantial rise in life expectancy and an aging population globally. This increase is anticipated to discriminately impact low- and middle-income populations the most, possibly increasing their risk of dementia by a factor of 4-5x in the next 25 years. As reviewed, no (widely accepted) treatments are available to directly target the underlying pathophysiology of MaNCD. With approximately 4 in 10 cases of dementia considered preventable, extraordinary focus must be placed on prevention and slowing disease progression.
Primary prevention of MaNCD focuses on delaying the disease onset by modifying lifestyle and behavioral risk factors.17,50 Estimates that a delay in the onset of dementia by 1% could reduce global rates of dementia by more than 10% in 205051 place all healthcare providers, including the HHCP, on the frontlines of prevention.
Prevention through lifestyle modifications may specifically target cognition in normal aging individuals, as well as improve cognition in persons with MiNCD. Currently, it is estimated that 15-20% of people with MiNCD transition to MaNCD each year.19 Unfortunately, with no intervention or modification of lifestyle, and given a long enough survival time, conversion from MiNCD to MaNCD may be inevitable.52
Each of the twelve modifiable lifestyle risk factors listed includes their population attributable factor (PAF) value (see Figure 2).
- Hearing Loss (8%) – encourage the early treatment of hearing loss for those affected and reduce exposure to excessive noise53-58
- Education (7%) – provide all people with the opportunity for lifelong learning, especially primary and secondary education59-62
- Smoking (5%) – avoid smoking63-66
- Depression (4%) – reducing depression may curb dementia neuropathology67-69
- Isolation (4%) – engage in more frequent social contact, especially during late middle age70-72
- Traumatic Brain Injury (3%) – prevent head injury73-76
- Physical Activity (2%) – sustain midlife and possibly later life physical activity77-78
- Reduce Hypertension (2%) – maintain systolic blood pressure of 130mmHG or less79-82
- Air Pollution (2%) – reduce exposure to air pollution and second-hand smoke83-85
- Diabetes (1%) – reduce rates of acquired diabetes (ie, Type-2 Diabetes)86,87
- Obesity (1%) – reduce obesity (as defined by BMI)88,89
- Alcohol Intake (1%) – Limit alcohol consumption to less than 21 units per week (the equivalent of 2 bottles of wine per week)90-93
Although not specifically assigned a PAF value by the Lancet Commission, sleep and diet were included as significant contributing factors to dementia risk. Consistently sleeping more than 6 hours per night is considered important for reducing the risk of decline and dementia.94-96 In addition, The World Health Organization guidelines recommend a Mediterranean diet (i.e., plant-based foods, whole grains, nuts, seeds, and moderate amounts of lean poultry and fish) to reduce the risk of cognitive decline or dementia, as it might help and does not harm.97
Discussion: Role of the HHCP in MaNCD
Rates of MaNCD exponentially increase every 5 years between the age of 65-90 years old,98 with the pre-clinical stage of disease beginning up to 20 years before symptoms manifest.99 The challenge facing the HHCP is that presbycusis often starts in the 4th to 5th decade of life,100 but the average age of the first-time hearing aid user is 74 years old.101 These pre-clinical and pre-symptomatic years for MaNCD and presbycusis are likely the most crucial years to successfully impact the trajectory of cognitive function and conversion from typical aging to MiNCD to MaNCD.
The HHCP is uniquely suited to screen their patient’s dementia risk as it relates to their information and auditory processing abilities,11 audiometric findings,13 and ability to hear and listen in complex listening environments.15 In recent years, many HHCPs have adopted formal cognitive screening measures,102 including dementia risk questionnaires, paper-based cognitive screenings, and sophisticated computerized cognitive screeners which do not depend on sound systems to deliver test questions, thereby eliminating tester bias, as well as eliminating tester and /or patient-based hearing or listening problems (as confounding sources of variability) and are automatically scored and a detailed report for the physician is provided.

Citation for this article: Darrow KN. Dementia and the hearing healthcare provider. Hearing Review. 2022;30(1):28-32.
References
- American Speech-Language-Hearing Association (ASHA) website. Code of Ethics. https://inte.asha.org/Code-of-Ethics/. Published March 1, 2016.
- The American Academy of Audiology (AAA). Code of Ethics of the AAA. Part I. Statement of principles and rules: Rule 1A, 1B. https://www.audiology.org/wp-content/uploads/2021/05/201910-CodeOfEthicsOf-AAA-1.pdf.
- Rabbitt PMA. Channel-capacity, intelligibility and immediate memory. Quar J Exp Psychol. 1968;20(3):241–248.
- Doherty KA, Desjardins JL. The benefit of amplification on auditory working memory function in middle-aged and young-older hearing-impaired adults. Front Psych. 2015;6(721):1-9.
- Qian ZJ, Wattamwar K, Caruana FF, et al. Hearing aid use is associated with better Mini-Mental State Exam performance. The American Journal of Geriatric Psychiatry. 2016;24(9):P694–P702.
- Chern A, Golub JS, Lalwani AK. Do hearing aids help prevent cognitive decline? The Laryngoscope. 2021;131(10):2166-2168.
- Weinstein BE. Hearing loss and senile dementia in the institutionalized elderly. Clinical Gerontologist. 1986;4(3):3-15.
- Uhlmann RF, Larson EB, Rees TS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261(13):1916-1919.
- Beck DL, Clark JL. Audition matters more as cognition declines. Cognition matters more as audition declines. Audiology Today. 2009:48-59.
- Powell DS, Oh ES, Reed NS, Lin FR, Deal JA. Hearing loss and cognition: What we know and where we need to go. Frontiers in Aging Neuroscience. 2022;13(769405):1-18.
- Beck DL. The emerging relationship between cognition and audition: Why cognitive screenings are beneficial for audiology patients and why comprehensive audiometric evaluations are recommended for people with mild cognitive impairment, cognitive decline and dementia. Journal of Otolaryngology-ENT Research. 2022;14(1):1-6.
- Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25(6):763–770.
- Lin FR, Metter J, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011;68(2):214-220.
- Lin FR, Yaffe K, Xiao J, et al. Hearing loss and cognitive decline among older adults. JAMA Intern Med. 2013;173(4):293-299.
- Stevenson JS, Clifton L, Kuźma E, Littlejohns TJ. Speech‐in‐noise hearing impairment is associated with an increased risk of incident dementia in 82,039 UK Biobank participants. Alzheimer’s & Dementia. 2022;18(3):445–456.
- Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):P2673-P2734.
- Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):P413-P446.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5-TR). 5th ed. American Psychiatric Publishing; 2013.
- Thaipisuttikul P, Jaikla K, Satthong S, Wisajun P. Rate of conversion from mild cognitive impairment to dementia in a Thai hospital-based population: A retrospective cohort. Alzheimer’s Dement. 2022; 8(1):e12272.
- Cohen JI, Gordon-Salant S. The effect of visual distraction on auditory-visual speech perception by younger and older listeners. The Journal of the Acoustical Society of America. 2017;141(5).
- Devere R. The cognitive and behavioral consequences of hearing loss: Part 1. Practical Neurology.2017;34-38.
- Rönnberg J, Hygge S, Keidser G, Rudner M. The effect of functional hearing loss and age on long- and short-term visuospatial memory: Evidence from the UK Biobank resource. Frontiers in Aging Neuroscience. 2014;6(326):1-13.
- Lodeiro-Fernández L, Lorenzo-López L, Maseda A, Núñez-Naveira L, Rodríguez-Villamil JL, Millán-Calenti JC. (2015, April 9). The impact of hearing loss on language performance in older adults with different stages of cognitive function. Clinical Interventions in Aging. 2015;10:695-702.
- Weinstein BE, Ventry IM. Hearing impairment and social isolation in the elderly. J Speech Hear Res. 1982;25(4):593-599.
- Duong S, Patel , Chang F. Dementia: What pharmacists need to know. Canadian Pharmacists Journal. 2017;150(2):118-129.
- Connors MH, Quinto L, Brodaty H. Longitudinal outcomes of patients with pseudodementia: A systematic review. Psychological Medicine. 49(5):727-737.
- Hshieh TT, Inouye SK, Oh ES. Delirium in the elderly. Psychiatr Clin North Am. 2018;41(1):1-17.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):939.
- McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):263–269
- Mahase E. Aducanumab: 4 in 10 high dose trial participants experienced brain swelling or bleeding. BMJ. 2021;375:n2975.
- Wang XX, Zhang B, Xia R, Jia QY. Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur Rev Med Pharmacol Sci. 2020;24(18):9601-9614.
- Engelhardt E, Tocquer C, André C, Moreira DM, Okamoto IH, Cavalcanti JLDS. Vascular dementia: Diagnostic criteria and supplementary exams: Recommendations of the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology. Part I. Dementia & Neuropsychologia. 2011;5(4):251-263.
- Benisty S, Hernandez K, Viswanathan A, et al. Diagnostic criteria of vascular dementia in CADASIL. Stroke. 2008;39(3):838–844.
- Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: An umbrella review of systematic reviews and meta-analyses. Alzheimers Dement. 2017;13(4):406-418.
- O’Brien JT, Taylor J-P, Thomas A, et al. Improving the diagnosis and management of Lewy body dementia: The DIAMOND-Lewy research programme including pilot cluster RCT. Programme Grants Appl Res. 2021;9(7).
- Aarsland D, Andersen K, Larsen JP, Lolk A. Prevalence and characteristics of dementia in Parkinson Disease: An 8-year prospective study. Arch Neurol. 2003;60(3):387–392.
- Hely MA, Reid WGJ, Adena MA, Halliday GM, Morris JGL. The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837-844.
- Nilsson C, Waldö ML, Nilsson K, Santillo A, Vestberg S. Age-related incidence and family history in frontotemporal dementia: Data from the Swedish Dementia Registry. PLOS ONE. 2014;9(4):e94901.
- Neary D, Snowden J, Mann D. Frontotemporal dementia. The Lancet Neurology. 2005;4(11):P771–P780
- Feldman H, Levy AR, Hsiung G-Y, et al. A Canadian cohort study of cognitive impairment and related dementias (ACCORD): Study methods and baseline results. Neuroepidemiology. 2003;22(5):265-274.
- Dooley M, Lamb HM. Donepezil: A review of its use in Alzheimer’s disease. Drugs & Aging. 2000;16:199-226.
- Zuidema SU, Johansson A, Selbaek G, et al. A consensus guideline for antipsychotic drug use for dementia in care homes. Bridging the gap between scientific evidence and clinical practice. Int Psychogeriatr. 27(11):1849–1859.
- Tampi RR, Tampi DJ, Balachandran S, Srinivasan S. Antipsychotic use in dementia: A systematic review of benefits and risks from meta-analyses. Therapeutic Advances in Chronic Disease. 2016;7(5):229-245.
- Bennett S, Thomas AJ. Depression and dementia: Cause, consequence or coincidence? Maturitas. 2014;79(2):P184‐P190.
- Leyhe T, Reynolds CF, Melcher T, et al. A common challenge in older adults: Classification, overlap, and therapy of depression and dementia. Alzheimer’s & Dementia. 2017;13(1):59‐71
- Federal Drug Administration (FDA) press release. FDA grants accelerated approval for Alzheimer’s drug. https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug. Published June 7, 2021.
- Sevigny J, Chiao P, Bussière T, et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016; 537:50-56.
- Vaz M, Silva V, Monteiro C, Silvestre S. Role of Aducanumab in the treatment of Alzheimer’s disease: Challenges and opportunities. Clin Interv Aging. 2022;17:797-810.
- Karlawish J, Jack CR, Rocca WA, Snyder HM, Carrillo MC. Alzheimer’s disease: The next frontier – Special report 2017. Alzheimers Dement. 2017;13(4):374-380.
- G8 Health and Science Ministers. G8 Dementia Summit Declaration. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/265869/2901668_G8_DementiaSummitDeclaration_acc.pdf. Published December 11, 2013.
- Peracino A. Hearing loss and dementia in the aging population. Audiol Neurootol. 2014;19(Suppl 1):6-9.
- Petersen RC, Negash S. Mild cognitive impairment: An overview. CNS Spectr. 2008;13(1):45-53.
- Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: A systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018;144(2): 115–126.
- Golub JS, Brickman AM, Ciarleglio AJ, Schupf N, Luchsinger JA. Association of subclinical hearing loss with cognitive performance. JAMA Otolaryngol Head Neck Surg. 2019;146(1):57–67.
- Armstrong NM, An Y, Doshi J, et al. Association of midlife hearing impairment with late-life temporal lobe volume loss. JAMA Otolaryngol Head Neck Surg. 2019;145(9):794-802.
- Amieva H, Ouvrard C, Meillon C, Rullier L, Dartigues J-F. Death, depression, disability, and dementia associated with self-reported hearing problems: A 25-year study. J Gerontol A Biol Sci Med Sci. 2018;73(10):1383–1389.
- Ray J, Popli G, Fell G. Association of cognition and age-related hearing impairment in the English Longitudinal Study of Ageing. JAMA Otolaryngol Head Neck Surg. 2018;144:(10):876–882.
- Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N. Longitudinal relationship between hearing aid use and cognitive function in older Americans. J Am Geriatr Soc. 2018;66(6):1130–1136.
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: An analysis of population-based data. Lancet Neurol. 2014;13(8):788–794.
- Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016; 374: 523–532. 37
- Kremen WS, Beck A, Elman JA, et al. Influence of young adult cognitive ability and additional education on later-life cognition. Proc Natl Acad Sci. 2019;116(6): 2021–2026.
- Van der Lee SJ, Teunissen CE, Pool R, et al. Circulating metabolites and general cognitive ability and dementia: Evidence from 11 cohort studies. Alzheimers Dement. 2018;14(6):707–722.
- Chang C-C, Zhao Y, Lee C-W, Ganguli M. Smoking, death, and Alzheimer disease: A case of competing risks. Alzheimer Dis Assoc Disord. 2012;26(4):300–306.
- Debanne SM, Bielefeld RA, Cheruvu VK, Fritsch T, Rowland DY. Alzheimer’s disease and smoking: Bias in cohort studies. J Alzheimers Dis. 2007;11(3):313–321.
- Choi D, Choi S, Park SM. Effect of smoking cessation on the risk of dementia: A longitudinal study. Ann Clin Transl Neurol. 2018;5(10):1192–1199.
- Almeida OP, Hankey GJ, Yeap BB, Golledge J, Flicker L. Depression as a modifiable factor to decrease the risk of dementia. Transl Psychiatry. 2017;7:e1117.
- Kelly ME, Duff H, Kelly S, et al. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: A systematic review. Syst Rev. 2017;6:259.
- Bartels C, Wagner M, Wolfsgruber S, Ehrenreich H, Schneider A. Impact of SSRI therapy on risk of conversion from mild cognitive impairment to Alzheimer’s dementia in individuals with previous depression. Am J Psychiatry. 2018;175(3):232–241.
- Sommerlad A, Ruegger J, Singh-Manoux A, Lewis G, Livingston G. Marriage and risk of dementia: Systematic review and meta-analysis of observational studies. J Neurol Neurosurg Psychiatry. 2018;89(3):231–238.
- Evans IEM, Martyr A, Collins R, Brayne C, Clare L. Social isolation and cognitive function in later life: A systematic review and meta-analysis. J Alzheimers Dis. 2019;70(S1):S119–S144.
- Penninkilampi R, Casey A-N, Singh MF, Brodaty H. The association between social engagement, loneliness, and risk of dementia: A systematic review and meta-analysis. J Alzheimers Dis. 2018;66(4):1619–1633.
- Zanier ER, Bertani I, Sammali E, et al. Induction of a transmissible tau pathology by traumatic brain injury. Brain. 2018;141(9): 2685–2699.
- Cao J, El Gaamouch F, Meabon JS, et al. ApoE4-associated phospholipid dysregulation contributes to development of tau hyper-phosphorylation after traumatic brain injury. Sci Rep. 2017;7:11372.
- Nordstrom A, Nordstrom P. Traumatic brain injury and the risk of dementia diagnosis: A nationwide cohort study. PLoS Med. 2018;15(1):e1002496.
- Yaffe K, Lwi SJ, Hoang TD, et al. Military-related risk factors in female veterans and risk of dementia. Neurology. 2019;92(3): e205–e211.
- Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D. Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. NeuroToxicology. 2017;61:143–187.
- Zotcheva E, Bergh S, Selbaek G, et al. Midlife physical activity, psychological distress, and dementia risk: The HUNT study. J Alzheimers Dis. 2018;66(2):825–833.
- Horder H, Johansson L, Guo X, et al. Midlife cardiovascular fitness and dementia: A 44-year longitudinal population study in women. Neurology. 2018;90(15):e1298–e1305.
- McGrath ER, Beiser AS, DeCarli C, et al. Blood pressure from mid- to late life and risk of incident dementia. Neurology. 2017; 89(24):2447–2454.
- Abell JG, Kivimaki M, Dugravot A, et al. Association between systolic blood pressure and dementia in the Whitehall II cohort study: Role of age, duration, and threshold used to define hypertension. Eur Heart J. 2018;39(33):3119–3125.
- Pase MP, Beiser A, Enserro D, et al. Association of ideal cardiovascular health with vascular brain injury and incident dementia. Stroke. 2016;47(5):1201–1206.
- Walker KA, Sharrett AR, Wu A, et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA. 2019;322(6):535–545.
- Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey KJ. Air pollution and dementia: A systematic review. J Alzheimers Dis. 2019; 70(1):S145–S163.
- Power MC, Adar SD, Yanosky JD, Weuve J. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and dementia: A systematic review of epidemiologic research. NeuroToxicology. 2016;56:235–253.
- Chen H, Kwong JC, Copes R, et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. The Lancet. 2017;389(10070):P718–P726.
- Chatterjee S, Peters SAE, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39(2):300–307.
- Sastre AA, Vernooij RWM, Harmand MG-C, Martinez G. Effect of the treatment of Type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst Rev. 2017;6:CD003804.
- Albanese E, Launer LJ, Egger M, et al. Body mass index in midlife and dementia: Systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst). 2017;8(1):165–178.
- Veronese N, Facchini S, Stubbs B, et al. Weight loss is associated with improvements in cognitive function among overweight and obese people: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;72:87–94.
- Rehm J, Hasan OSM, Black SE, Shield KD, Schwarzinger M. Alcohol use and dementia: A systematic scoping review. Alzheimers Res Ther. 2019;11(1).
- Schwarzinger M, Pollock BG, Hasan OSM, Dufouil C, Rehm J. Contribution of alcohol use disorders to the burden of dementia in France 2008–13: A nationwide retrospective cohort study. Lancet Public Health. 2018;3(3):e124–e132.
- Sabia S, Fayosse A, Dumurgier J, et al. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ. 2018;362:k2927.
- Topiwala A, Allan CL, Valkanova V, et al. Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: Longitudinal cohort study. BMJ. 2017;357:j2353.
- Sindi S, Kåreholt I, Johansson L, et al. Sleep disturbances and dementia risk: A multicenter study. Alzheimer’s & Dementia. 2018;14(10):1235-1242.
- Spira AP, Gamaldo AA, An Y, et al. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70(12):1537–1543.
- Macedo AC, Balouch S, Tabet N. Is sleep disruption a risk factor for Alzheimer’s disease? J Alzheimers Dis. 2017;58(4): 993–1002.
- Pistollato F, Iglesias RC, Ruiz R, et al. Nutritional patterns associated with the maintenance of neurocognitive functions and the risk of dementia and Alzheimer’s disease: A focus on human studies. Pharmacol Res. 2018;131:32–43.
- Jorm AF, Jolley D. The incidence of dementia: A meta-analysis. Neurology. 1998;51(3):728–733
- Dubois B, Hampel H, Feldman HH, et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s & Dementia. 2016;12(3):292-323.
- Wang M, Ai Y, Han Y, Fan Z, Shi P, Wang H. Extended high-frequency audiometry in healthy adults with different age groups. J of Otolaryngol – Head & Neck Surg. 2021;50(52).
- McCormack A, Fortnum H. Why do people fitted with hearing aids not wear them? International Journal of Audiology. 2013;52(5):360-368.
- Davis, J. Cognitive screening in audiology: Considerations for nonverbal instructions. The Hearing Journal. 2021;74(5):34-35.
- Kochkin S, Rogin C. Quantifying the obvious: The impact of hearing instruments on quality of life. Hearing Review. 2000;7(1):6-34.
- Brenowitz WD, Wallhagen MI. Does hearing impairment affect physical function? JAMA Netw Open. 2021;4(6):e2114782.
- Kuo P-L, Di J, Ferrucci L, Lin FR. Analysis of hearing loss and physical activity among us adults aged 60-69 years. JAMA Netw Open. 2021;4(4):e215484.
- Martinez-Amezcua P, Powell D, Kuo P-L, et al. Association of age-related hearing impairment with physical functioning among community-dwelling older adults in the US. JAMA Netw Open. 2021;4(6):e2113742.
- Martinez-Amezcua P, Kuo P-L, Reed NS, et al. Association of hearing impairment with higher-level physical functioning and walking endurance: Results from the Baltimore Longitudinal Study of Aging. J Gerontol Series A. 2021;76(10):e290-e298.
- Mener DJ, Betz J, Genther DJ, Chen D, Lin FR. Hearing loss and depression in older adults. J Am Geriatr Soc.2013;61(9):1627-1629.





This Special Edition of Hearing Review (HR) on cognition is one of the best I have read! Congratulations to each author who contributed. Dr. Darrow, your article was jam-packed with a lot of new informative information. Thank you! This kind of information for practicing audiologists and the integration into audiology school curricula will help expand audiologist’s scope of practice and strengthen the profession’s position as point-of-entry doctors in health care for audio-vestibular disorders.
I do have a request going forward regarding the use of the misidentifying phrase ‘hearing healthcare provider’ (HHCP). My request is for the HR editors and future authors to discontinue using it or any derivation thereof. Respectfully, here’s why:
The importance and usage of correct terminology cannot be over-emphasized. The HHCP phrase started appearing in the National Academy of Sciences’ (NAS) 2016 report related to over-the-counter hearing aids. The NAS report reads, in part, “For the purposes of this report the term “hearing health care professionals” is used broadly to encompass those who work in hearing healthcare (including audiologists, hearing instrument specialists, and otolaryngologists). The term is used throughout the report primarily for ease – that is, one collective term, rather than listing each group repeatedly throughout the report”.
Identification and recognition of separate occupations, i.e., hearing aid dispensers (HADs) and professions, i.e., physicians and audiologists, are essential for consumer understanding and transparency. Neither expediency nor convenience should be accepted as a rationale to blur the lines between them. Referring to these groups in a generic “one size fits all” manner and as a collective of HHCPs will serve only to confuse and ultimately mislead consumers. Out of respect, their separate and very different identities should not be eliminated.
Also, continued use and reference to HHCP is to imply that HADs also begin to screen and test for cognitive impairment. Statutorily, this is not a viable option. HAD’s licensing laws authorize them to ‘test for the purpose of fitting hearing aids’. Any type of ‘screening’ mentioned in their licensing statutes is commonly referenced to ‘hearing screening’ as a HAD might provide at state fairs. Promoting and encouraging HADs to go beyond their ‘duties and responsibilities’ as outlined in their licensing laws is to promote HADs to practice audiology and/or medicine without a license. Similarly, HADs are not allowed to evaluate and treat for tinnitus, auditory processing disorders, vestibular disorders, etc.