Nearly a decade of work has demonstrated that both term and premature neonates show non-adult-like distortion product otoacoustic emission (DPOAE) suppression tuning and suppression growth. However, DPOAE suppression has not been measured beyond the newborn period. Therefore, the exact time course for maturation of cochlear function as measured by this paradigm is still unknown. In this experiment, DPOAE suppression was recorded in 3-month-old infants, as well as term neonates and adults (f2=6000 Hz). Results indicate that DPOAE suppression tuning is still not adult-like by 3 months of age. Term neonates and 3-month-olds have comparable DPOAE suppression results and adults are dissimilar from either infant group. This result suggests continued maturation of cochlear function in humans during the post-natal months.
In the past two decades, it has become apparent that sound-induced motion of the basilar membrane within the cochlea is augmented by intrinsic active processes. Auditory sensitivity and frequency resolution are enhanced because these processes,—collectively termed the “cochlear amplifier”—work to augment basilar membrane vibration only near the characteristic frequency or peak of the traveling wave evoked on the basilar membrane. This amplification process is only effective at low sound levels and saturates at moderately high stimulus levels. The motile properties of the outer hair cells provide energy for enhancement of basilar membrane motion.1 A recently discovered motor protein, called “prestin,” found in the outer hair cell (OHC) lateral membrane is required for normal OHC movement and cochlear amplifier function.2,3
Otoacoustic emissions (OAEs) are normal by-products of cochlear amplifier function and their existence clearly establishes the cochlea as a nonlinear mechanism. They provide a convenient metric for evaluating the integrity of this active process within the cochlea. Distortion product OAEs (DPOAEs) are pure tones produced by the cochlea when the ear is presented with two simultaneous tones. The cochlea, in turn, creates a third tone (the distortion product) at frequencies mathematically related to the two stimulating tones or “primary tones” (f1, f2). The most robust of these distortion products is at 2f1-f2. Thus, DPOAEs reflect outer hair cell integrity and provide a non-invasive paradigm to study the maturation and development of cochlear function in human newborns.
Figures 1a-b. A series of suppression growth functions recorded from a normal-hearing adult. The upper panel in Figure 1a displays suppression growth for tones lower than f2 (fs<f2). The lower panel of Figure 1a displays suppression growth for tones higher than f2 (fs>f2). The orange asterisk (*) represents the unsuppressed DPOAE level and the dashed line is set at 6 dB of amplitude suppression. 1b) Example of an STC derived from the suppression growth series in Figure 1a.
For the last decade, scientists at the HEI laboratory have studied cochlear development of preterm and term-born neonates. We have used various paradigms but focused our work primarily on ipsilateral DPOAE suppression. DPOAEs can be suppressed by a third tone (fs) presented simultaneously with f1 and f2. DPOAE suppression tuning curves (STCs) are generated by presenting several suppressor tones of varying frequency (centered around f2) together with the two primary tones. Each suppressor tone is increased in level until the DPOAE is reduced in amplitude by a criterion amount, typically 6 dB. Figure 1a shows a series of suppression growth functions. These graphs display the amplitude of the DPOAE as a function of increasing suppressor level. As the suppressor level increases, the DPOAE amplitude decreases. Each line represents the unique pattern and rate of suppression evoked by different frequency suppressor tones. Both suppressor tones below (upper panel) and above (lower panel) f2 are presented and suppression grows at varying rates depending on frequency. Figure 1b displays the STC that was generated by plotting the level of suppressor tone required to achieve 6-dB suppression, as a function of suppressor frequency.
Over the past 7-8 years, DPOAE suppression experiments conducted to study cochlear maturation, have led to the following findings:
1) Premature neonates have narrower suppression tuning curves, with steeper low-frequency flank, than adults at f2 frequencies of 1500 and 6000 Hz. The most pronounced age effect is at 6000 Hz;
2) When low-frequency suppressor tones are presented to premature neonates, their DPOAEs are harder to suppress. Thus, the growth of suppression is shallower and more compressive.
DPOAE suppression data collected recently in our lab using a longitudinal design has shown that prematurely born neonates that have reached “term-like” age (39-41 weeks post-conceptional age [PCA]) continue to show these immaturities. Even term-born neonates show non-adult-like DPOAE suppression.4 This suggests that the cochlea continues to develop post-natally.
We do not know, however, when the cochlear amplifier—as measured by DPOAE suppression—becomes completely adult-like. For this reason, studies of suppression in “older” infants must be conducted to answer this question. In the present experiment, we studied DPOAE suppression at 6000 Hz in 3-month-olds, term newborns, and normal-hearing adults. The inclusion of this older infant age group allows us to study the post-natal period of cochlear development. By comparing DPOAE suppression results in older infants to the results of newborns and adults, it will help us define the time-course for the maturation of cochlear function.
Methods
Subjects: Ten term neonates (1 female, 9 males), 9 normal-hearing 3-month-old infants (4 females, 5 males), and 8 adults (7 females, 1 male) were used as subjects in this study. All adults had audiometric thresholds <15 dB HL between 500 and 8000 Hz and negative history of otologic pathology.
The 19 infant subjects were born at Women and Children’s Hospital, Los Angeles County-University of Southern California Medical Center between 38-41 weeks of gestation. All neonates passed a hearing screening (30 dB HL click-evoked ABR) prior to inclusion into this study and none had high-risk factors for hearing loss.5 The 10 term neonates were tested within 72 hours of birth. The nine 3-month-old infants were tested in the infant auditory lab at ages ranging between 3 and 4 months of age.
Instrumentation and Signal Analysis: An Ariel DSP16+ signal processing and acquisition board housed within a Compaq Prolinea 590 personal computer with Pentium processor was used to generate stimuli and acquire data. The Ariel board was connected to an Etymotic Research ER-10C probe system. The ER-10C probe contains 2 output transducers and a low-noise microphone.
Data acceptance criteria were as follows: 1) Noise measurements for 3 frequency bins (12.2 Hz wide) on either side of the 2f1-f2 frequency had to be < 0 dB SPL to assure appropriate subject state, and 2) The measured DPOAE level had to be at least 5 dB above the average noise measured in the same 6 bins around the distortion product frequency to be accepted into the grand average.
Procedure: Adult subjects were tested in a sound-treated booth at the House Ear Institute while reading or sitting quietly. Infants were tested within the hospital. The neonates were fed, if necessary, prior to the test, swaddled, and placed in an isolette to sleep. The 3-month-old infants were tested in an isolette, car seat, or their mother’s arms.
Custom-designed software for the collection of DPOAE suppression data was developed at the House Ear Institute. DPOAE suppression tuning curves (STCs) were recorded at f2=6000 Hz only. The most robust age effects for DPOAE suppression have been previously observed at 6000 Hz. Therefore, this test frequency was chosen to enhance the probability of detecting age-related effects. DPOAEs were recorded with an f2/f1 ratio of 1.2 and moderate (65-55 dB SPL) primary tone levels.
An unsuppressed DPOAE was initially recorded. A suppressor tone (fs) of a given frequency was then presented simultaneously with the primary tones, and its level increased in 5 dB steps over a range of intensities from 40 to 85 dB SPL. Fifteen suppressor tones with frequencies ranging from 1-octave below to 1/4-octave above 6000 Hz were presented at intervals between 25-150 cents (one octave = 1200 cents). An unsuppressed DPOAE was recorded before each new suppressor tone was presented. This process resulted in a family of suppression growth functions (DPOAE amplitude x suppressor level) similar to those shown in Figure 1a. To generate DPOAE STCs, the suppressor level that reduced DPOAE amplitude by 6 dB was determined for each suppressor tone using linear interpolation and then plotted as a function of its frequency. In Figure 1a, the dotted horizontal line indicates the suppressor level at which the DPOAE was reduced by 6 dB.
Data Analysis
Suppression Tuning Curves: Suppression tuning curves were analyzed in the following manner: (1) tuning curve width was quantified by measuring Q10, an index of tuning sharpness, (2) slope of the low- and high-frequency flank of the tuning curve was determined with a regression equation and (3) tuning curve tip frequency and level were measured. Statistical analyses of Q10, low- and high frequency tuning curve slope and tip characteristics were conducted among groups to test for age effects.
Figure 2. Calculation of suppression growth slope (dB/dB) for two suppressor tones. A slope value was obtained for each suppressor tone by fitting a regression equation to the function (see white line for fit). The resulting slope value reflects the rate at which the suppression grows as the level is increased. A high-frequency suppressor produces shallow growth of suppression (0.2 dB/dB) while a low-frequency suppressor produces steep growth (1.3 dB/dB).
Suppression Growth: Suppression growth was determined for each subject by fitting a regression equation to each suppression growth function as shown in Figure 2 (see white lines for fit). Therefore, a slope value was obtained for each suppressor tone, reflecting the unique rate of DPOAE suppression growth each tone produced as its level was increased. Figure 2 provides an example of a suppression growth function showing steep suppression growth (1.3 dB/dB) and one showing shallow growth (.2 dB/dB).
Figure 3. Suppression growth slope as a function of suppressor frequency. The dashed vertical line is placed at the f2 frequency; thus, data points to the left of the line are produced by suppressor tones lower in frequency than f2; conversely, data points to the right of the line are from suppressor tones higher than f2. Along the Y-axis, red slope values represent steep growth of suppression and green values represent shallow growth of suppression.
To compare DPOAE suppression growth among ages, slope was plotted as a function of suppressor frequency for each subject and each age group. Figure 3 provides an example of the normal pattern of suppression growth across frequency. The red slope values (>1.0) reflect steep growth and the green values indicate shallow growth (<1.0) (see Y-axis). Each blue data point reflects a slope value plotted as a function of suppressor frequency. It is typical for suppressors lower than f2 (those plotted to the left of the vertical dashed line) to produce steep slope of suppression growth; suppressors higher than f2 (to the right of the vertical dashed line) produce shallow suppression growth. This well-established pattern of suppression is based in the recognized non-linearity of basilar membrane motion for tones above and below a given reference or probe frequency (in this case, f2).
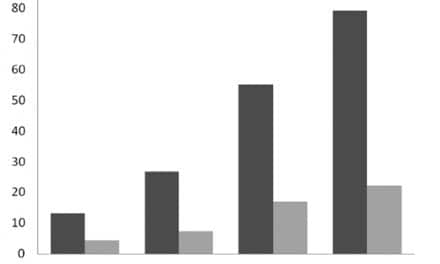
Figure 4. Mean DPOAE STC width (Q10) values for three age groups: adults, term neonates, and 3-month-old infants. Term neonates and 3-month-old infants had comparable Q10 values that were significantly larger than adult values. This indicates sharper DPOAE suppression tuning for infants.
Results
Suppression Tuning Curves: Mean tuning curve width (Q10) values for term neonates, 3-month-old infants, and adults are presented in Figure 4. The mean Q10 values were as follows: term neonates = 3.27; 3-month-olds = 2.97; adults = 2.23. Both the term group and the 3-month-old infants had significantly larger Q values (indicating narrower tuning) than adults. However, tuning curve width was not significantly different between the 2 infant groups. Although non-adult-like, the mean Q10 value from the 3-month-old group appears to be moving in an adult-like direction.
Figure 5. Mean slope on the low-frequency flank of the DPOAE STC for three age groups: adults, term neonates, and 3-month-old infants. Term neonates and 3-month-old infants had comparable low-frequency slope. Tuning curve slope was steeper for infants than adults.
An age effect was also evident for the low-frequency flank of the STC (see Figure 5). DPOAE STCs from 3-month-olds and term neonates show steeper low-frequency flank (larger dB/octave values) than adult tuning curves indicating sharper tuning. Older infants and term neonates were not significantly different from one another. The STC high-frequency slope data are not shown because values from adults and infants show complete overlap. STC tip features did not show any significant age effects. Both the frequency at which the STC tip is centered and the suppressor level comprising the tip are comparable among age groups.
Figure 6. Mean slope of suppression growth for 15 suppressor tones ranging from 3620 Hz to 7239 Hz. Mean data are shown for adults, term neonates, and 3-month-old infants. Both infant groups have shallower, more compressive growth of suppression than adults at the lowest-frequency suppressor tones.
Suppression Growth
The typical adult pattern of suppression growth was not observed in term or 3-month-old infants. Previous results have shown that neonates do not have characteristically steep growth of suppression for low-frequency suppressor tones. The present results extend this finding to 3-month-old infants. As shown in Figure 6, both infant groups have more shallow suppression growth for the lowest suppressor tones (left of vertical line). Although non-adult-like, 3-month-old DPOAE suppression growth falls between adult and term neonates, suggesting that these older infants may be moving toward adult-like values.
Summary of Results
Term neonates are similar to 3-month-old infants with respect to STC width, slope, and suppression growth. Neither infant group is adult-like at the test frequency (f2 = 6000 Hz) used in this experiment. This finding suggests a subtle immaturity in cochlear function that persists into the post-natal period. Future studies of cochlear function in humans should target the first 6 months of post-natal life to fully define the maturational process.
Discussion
Previous work has clearly shown that premature neonates have non-adult-like cochlear function as measured by DPOAE suppression. More recently, experiments using a longitudinal design to track maturational changes within individual subjects have shown that even term-born newborns are not completely adult-like in DPOAE suppression characteristics. These results indicated to us that we needed to investigate the early post-natal period. For this reason, the present study measured DPOAE suppression in a novel, older infant group, (ie, 3-month-olds).
The DPOAE suppression results from term newborns were not unexpected since previous reports have indicated that tuning is narrower and low-frequency suppression growth is shallower in these subjects. The new finding is that 3-month-old infants are similar to newborns and dissimilar from adults with respect to their DPOAE suppression characteristics. This finding supports the hypothesis that cochlear maturation is continuing into the early post-natal period and strongly suggests that future studies extend their time period of investigation through six months of age. It is unlikely that the cochlea shows even subtle immaturity beyond this age since central measures of tuning (ABR and psychoacoustic tuning curves) are mature by six months.6,7
The underlying source or basis for immaturity of DPOAE suppression in infants as old as 3 months of age is not well understood. Because outer hair cells (OHCs) are morphologically mature very early in gestation (22 weeks PCA), the deficits we are recording with our DPOAE tests must come from very subtle dysfunction rather than gross abnormality in sensory cell morphology or cochlear anatomy. In other reports, we have hypothesized that the subtle cochlear immaturity contributing to these DPOAE findings may involve regulation of the cochlear amplifier and/or its’ boundaries of function. That is, OHCs may look mature and be generally functional, but they may not have precise boundaries of function. They may show some inaccuracy in movement and/or transition from resting point to motility. A second possibility is that OHC motility is completely mature, but the descending efferent fibers of the medial olivocochlear system (which synapse almost exclusively on the OHCs) are not quite mature. This system generally exerts an inhibitory response on OHCs. If this system is not adult-like and has some jitter or imprecision in it, the cochlear amplifier and the by-products it produces might reflect this imprecision.
OHC Motility: The first hypothesis has not been tested. We do not know if immaturities in OHC motility are contributing to the findings. Likewise, we do not know if the now-familiar constellation of DPOAE suppression results in neonates (ie, narrower than typical STCs, steeper low-frequency flank, and shallow growth of suppression) is due to some kind of immaturity in how the OHCs move. In laboratory animals, correlations have been found between the development of the OHC lateral membrane and development of OHC electromotile properties in vitro.8 As the lateral membrane of the OHC develops, electromotility develops. Electromotility of the OHC also appears to develop concurrently with the post-natal development of prestin immunoreactivity, suggesting that prestin is, in fact, the OHC motor protein.9 These changes in the OHC motility mechanism develop around the onset of hearing in both rats and gerbils.8,10
In humans, it is not clear when OHC electromotility develops; however, if the results from various small mammals are considered, it would appear to happen sometime after the onset of hearing (about 28 gestational weeks in humans). This suggests that maturation of OHC motility might continue into the post-natal period in humans, but at present we have no evidence to support this. Our laboratory is conducting a study in the near future to test this hypothesis by temporarily disabling OHC motility in human adults who have taken salicylates (aspirin) and then recording their DPOAE suppression.
Medial Efferent System: The second hypothesis is that regulation of OHC motility and cochlear amplifier function is immature. This implicates the medial efferent system. The first appearance of medial olivocochlear (MOC) neurons in the human brainstem and at the base of the OHCs occurs at approximately 20-22 fetal weeks.10-12 However, changes in OHC innervation by MOC fibers have been observed late into the third trimester and may continue into the early post-term weeks and months.10-11
Work from our laboratory has shown that contralateral noise does not evoke an adult-like MOC response in premature neonates.13 Other investigators have been unable to detect MOC-mediated contralateral suppression of the click-evoked OAEs in premature neonates.14-16 It appears that the medial efferent influence on OHC function (as measured by contralateral suppression of OAEs) is approaching adult-like status around term birth but may not be completely mature at this time.
Why is the MOC important to cochlear function and maturity? The exact function of the efferent system is not known. But, in general, the MOC system has an inhibitory effect on the auditory periphery. The medial olivocochlear system appears to be involved in the stabilization of OHC motility, among other things, and thus constitutes a control system of sorts for the cochlear amplifier.17
Some have hypothesized that the medial olivocochlear fibers regulate OHC length/tension and, consequently, maintain an optimal operating point for fast motile activity.18 Others suggest that efferent fibers provide constant adjustment of the dynamic range of hearing and provide the precise amount of amplification required for a given acoustic situation.19 The MOC may maintain the cochlea at an optimum mechanical state for efficient function of active processes.20
It is not difficult to see that, if this crucial “control” or regulatory function is absent or immature in neonates, the cochlear amplifier might produce DPOAE results that aren’t quite adult-like—perhaps similar to those we observe in our infant subjects.
Summary
It is now clear that DPOAE characteristics are not completely adult-like in human infants at f2 = 6000 Hz—even after they reach term or term-like status. This strongly suggests that maturation of cochlear function is achieved sometime after the time period associated with normal gestation. Furthermore, the present study extends this finding to suggest that, even by 3 months of age, residual immaturity of DPOAE suppression characteristics is present.
The timeline and sequence for development of sensory cell morphology and cochlear anatomy indicates that a residual immaturity in cochlear function would be subtle and might involve some “jitter” or regulatory imprecision rather than gross dysfunction. Further work is warranted to fully define the timeline and to investigate the underlying sources of these immature responses. Our laboratory is currently initiating and will begin implementation of these projects in the next year.
Acknowledgements
The authors would like to acknowledge Dr. Ellen Ma for data collection. This study was funded by a grant from the National Institutes of Health, NIDCD R29 DC03552 and by the House Ear Institute.


|
References
1. Dallos P. The active cochlea. J Neuroscience. 1992; 12:4575-4585.
2. Liberman M, Gao J, He D, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;19:300-304.
3. Zheng J, Madison L, Oliver D, Fakler B, Dallos P. Prestin, the motor protein of outer hair cells. Audiol Neurootol. 2002;7, 9-12.
4. Abdala C. A longitudinal study of DPOAE ipsilateral suppression and input/output characteristics in human neonates. J Acoust Soc Am. 2003; In press.
5. Gerkin K. The high-risk register for deafness. ASHA. 1984;26,17-23.
6. Abdala C, Folsom R. Frequency contribution to the click-evoked auditory brainstem response in human adults and infants. J Acoust Soc Am. 1995;97:2394-2404.
7. Spetner N, Olsho L. Auditory frequency resolution in human infancy. Child Dev. 1990; 61:632-652.
8. He D, Evans B, Dallos P. First appearance and development of electromotility in neonatal gerbil outer hair cells. Hear Res. 1994;78:77-90.
9. Belyantseva I, Adler H, Curi R, Frolenkov G, Kachar B. Expression and localization of prestin and the sugar transporter GLUT-5 during development of electromotility in cochlear outer hair cells. J Neuroscience. 2000; 20:RC116 (1-5).
10. Pujol R, Carlier E, Lenoir M. Onogenetic approach to inner and outer hair cell functions. Hear Res. 1980;2:423-430.
11. Lavigne-Rebillard M, Pujol R. Hair cell innervation in the fetal human cochlea. Acta Otolaryngol. 1988;105:398-402.
12. Moore J, Simmons D, Guan Y-L. The human olivocochlear system: Organization and development. Audiol Neurootol. 1999; 4:311-325.
13. Abdala C, Ma E, Sininger Y. Maturation of medial efferent system function in humans. J Acoust Soc Am. 1999;105:2392-2402.
14. Goforth L, Hood L, Berlin C. Efferent suppression of transient-evoked otoacoustic emissions in human infants. In: Abstr. from the Assoc Res Otolaryngol., St Petersburg Beach, FL, 1997; Vol. 20:166.
15. Morlet, T, Collet L, Salle B, Morgon A. Functional maturation of cochlear active mechanisms and of the medial olivocochlear system in humans. Acta Otolaryngol. 1993;113:271-277.
16. Ryan S, Piron J. Functional maturation of the medial efferent olivocochlear system in human neonates. Acta Otolaryngol. 1994; 114:485-489.
17. Maison S, Micheyl C, Chays A, Collet L. Medial olivocochlear system stabilizes active cochlear micromechanical properties in humans. Hear Res. 1997;113,89-98.
18. Kim D. Active and nonlinear cochlear biomechanics and the role of outer-hair cell subsystem in the mammalian auditory system. Hear Res. 1986;22:105-114.
19. Guinan J. Physiology of olivocochlear efferents. In: Dallos P., Popper A, Fay R, eds. The Cochlea. New York: Springer-Verlag; 1996:435-502.
20. Johnstone B, Patuzzi R, Yates G. Basilar membrane measurements and the traveling wave. Hear Res. 1986;22:147-153.
Correspondence can be addressed to HR or Caroline Abdala, PhD, House Ear Institute, Children’s Auditory Research and Evaluation Center, 2100 West Third Street, Los Angeles, CA 90057; email: [email protected].

.gif)
.gif)
.gif)
.gif)
.gif)
.gif)
.gif)
.gif)