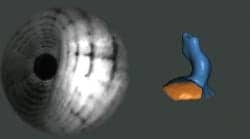
The Lantos system replaces the current laborious and often uncomfortable process of taking a silicone impression for the production of custom-fit ear devices.
Jeffrey Leathe, CEO and chairman of the board at Lantos, commented in the press release, “We are 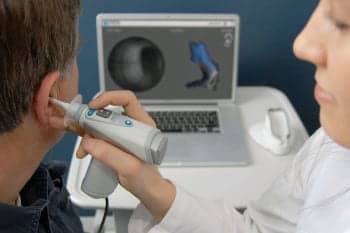
excited to receive FDA 510(k) clearance; it demonstrates our commitment to offer innovative and disruptive technology that is both safe and efficacious.”
The 3D Ear Scanning System also promises manufacturing efficiencies, simplified logistics, and improved turnaround time for product delivery to the customer.
The company says that the 3D Ear Scanning System will be commercially available later this year.
See related article in the HR’s Archives: Making a Digital Impression Using 3D Ear Canal Scanning.
SOURCE: Lantos Technologies



