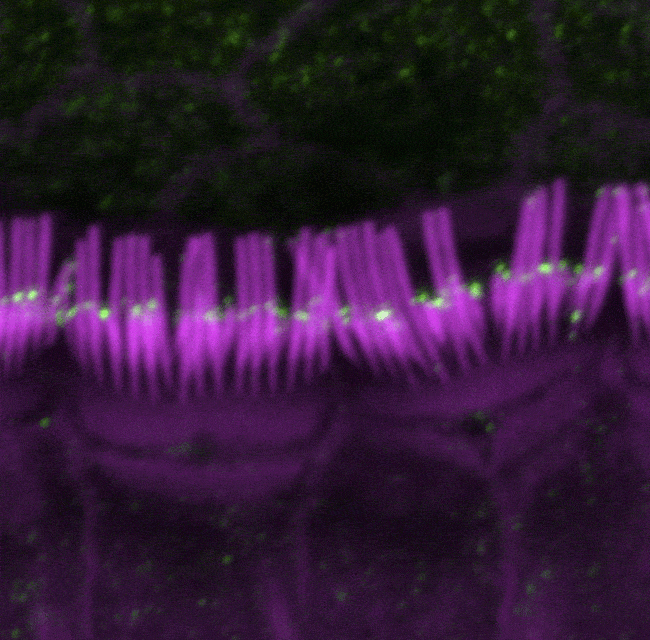
The large isoform of myosin 15 (green) localizes predominately at the tips of short stereocilia (purple), but not tall stereocilia, in inner hair cells in the cochlea of mice.
A research team led by scientists at the National Institute on Deafness and Other Communication Disorders (NIDCD), part of the National Institutes of Health (NIH), has discovered that a protein essential for building key hearing structures in the inner ear also plays a critical role in maintaining them throughout life. According to an announcement from the NIDCD, this discovery could lay the foundation for new approaches to preserve hearing and prevent or minimize the most common forms of hearing loss.
In a study article that appears in the October 6, 2015 edition of the journal eLife, and is also highlighted in an “Insight” article in the same journal, the researchers report that healthy hearing involves two distinct forms of a molecular motor protein called myosin 15 (MYO15A)—one form that helps build stereocilia, and a second, much longer, version of the protein that is needed to maintain stereocilia. Stereocilia are the finger-like projections that extend from the surface of hair cells, the inner ear’s sensory cells.
The study builds upon decades of research by NIDCD scientists and others to understand the formation and function of stereocilia. Stereocilia are like antennae, sensing sound waves passing through the ear and converting them to electrical signals that are transmitted to the brain. They are arranged in rows of increasing length on the hair cell surface, forming a staircase-like structure that is critical to their function.
Researchers have previously shown that some mutations in myosin 15 stunt stereocilia growth and prevent them from reaching their normal height, causing profound deafness. Scientists believe that myosin 15, which like other motor proteins can move around the cell, helps to build stereocilia by delivering critical component parts.
The current study was reportedly prompted by an earlier discovery of an unusual, deafness-associated mutation in the myosin 15 gene in a large Pakistani family. Previously studied myosin 15 mutations lie in the latter half of the gene, but this mutation lies toward the front of the gene. The affected family members did not have as substantial a hearing loss as expected based on work with the earlier mutations. This difference led the scientists to question if there were two forms of myosin 15, each with a separate function in the inner ear.
The structure of the myosin 15 gene also suggested to the researchers that it could produce two forms of the protein—a full-length form and a shorter form, lacking the front end. The novel mutation would only affect the longer version, leaving the shorter one intact. This could explain why the associated deafness was slightly less severe than for the previously studied mutations, which would damage both forms.
To further explore the possibility that there are two versions of myosin 15 involved in hearing, the researchers altered the gene in mice to have the same mutation as the unusual one found in the Pakistani family. Like their human counterparts, these mice produced the shorter form but lacked the longer one. By comparing them to normal mice and to mice missing both forms, the researchers were able to distinguish any unique functions associated with each version.
The study results revealed that while the shorter version of myosin 15 is sufficient for the construction of the stereocilia staircase, the longer version plays a critical role in maintaining it. Imaging studies showed that the shorter form is produced predominantly early in development throughout the staircase, while the longer form is largely made later and localizes to the shorter rows, providing further support for their distinct roles. Together, the findings show that myosin 15, through its dual forms, not only helps construct the stereocilia staircase of hair cells, but also works to maintain them, a process that until now has been poorly understood.
The research study was a collaboration between the NIDCD, the University of Michigan Medical School Department of Human Genetics, and the University of Kentucky. Jonathan Bird, PhD, is the communicating author and a postdoctoral fellow in the NIDCD Laboratory of Molecular Genetics.
Source: NIDCD, NIH
Photo credits: Jonathan E. Bird, Thomas B. Friedman, NIH, NIDCD