Tech Topic | February 2023 Hearing Review
Lenire helped participants to feel more confident and optimistic about their tinnitus management.
By Tish Ramirez AuD, Caroline Hamilton BSc, MSc, Sook Ling Leong PhD
In the May 23, 2022 online issue of Hearing Review,1 we discussed exit interview findings from the first Neuromod Devices large-scale clinical trial, TENT-A1 (Treatment Evaluation of Neuromodulation for Tinnitus-Stage 1), clinical trials,2 regarding the usability of Lenire®, which is a non-invasive bimodal (sound and tongue) stimulation portable device that is CE marked in Europe for use in the treatment of chronic, subjective tinnitus. In brief, TENT-A1 study participants found the Lenire® treatment device comfortable to use and highly practical with a treatment regime that can be fitted into their daily lives.1
In the present article, we will compare and discuss the user experience of Lenire in TENT-A1 to responses from Neuromod’s second clinical trial, TENT-A2 (Treatment Evaluation of Neuromodulation for Tinnitus-Stage 2), clinical trials.3 In addition, we will explore how the Lenire treatment impacted various aspects of the daily lives, including quality of life, for study participants across both studies.
TENT-A2 design and key outcomes
Based on the findings from the TENT-A1 study,2 a subsequent clinical trial called TENT-A2, was carried out to investigate and answer several questions that arose from TENT-A1, including the effects of different sound and tongue stimulation components on therapeutic outcomes and the effects of adjusting different stimulation settings to further improve tinnitus symptoms over time. The study consisted of four treatment arms with different parameter settings or components evaluated over a 12-week treatment period with a post-treatment follow-up period of 12 months.3
TENT-A23 showed that changing the stimulation settings over time provided enhanced clinical benefits to participants. The majority of participants exhibited a clinically meaningful reduction in symptoms during the first 6-weeks, which was further improved during the second 6-weeks of treatment by changing the stimulation settings. The study further showed that significant improvements in tinnitus could be achieved with tones paired with tongue stimulation with or without the inclusion of background noise stimuli.
Consistent with TENT-A1,2 there were no treatment-related serious adverse events in the TENT-A2 study.3 As in the TENT-A1 study, during the final visit of the TENT-A2 study, an exit interview was conducted to obtain qualitative feedback from participants about their experience during the clinical trial. As noted in the published Nature Scientific Reports manuscript3 and similar to that in TENT-A1,2 the tolerability of the treatment was found to be very high – 83.8% of participants that commenced the treatment were compliant with the treatment protocol. Also, a larger percentage of participants reported a high level of satisfaction with the treatment device in TENT-A2 compared to TENT-A1 (70.3% vs. 66.5%), who said they benefited from using the device based on the exit interview. A higher percentage of participants in TENT-A2 compared to TENT-A1 (87.8% vs. 77.8%) also reported that they would recommend the treatment to someone with tinnitus.
Comparable utility and usability experiences in both clinical trials
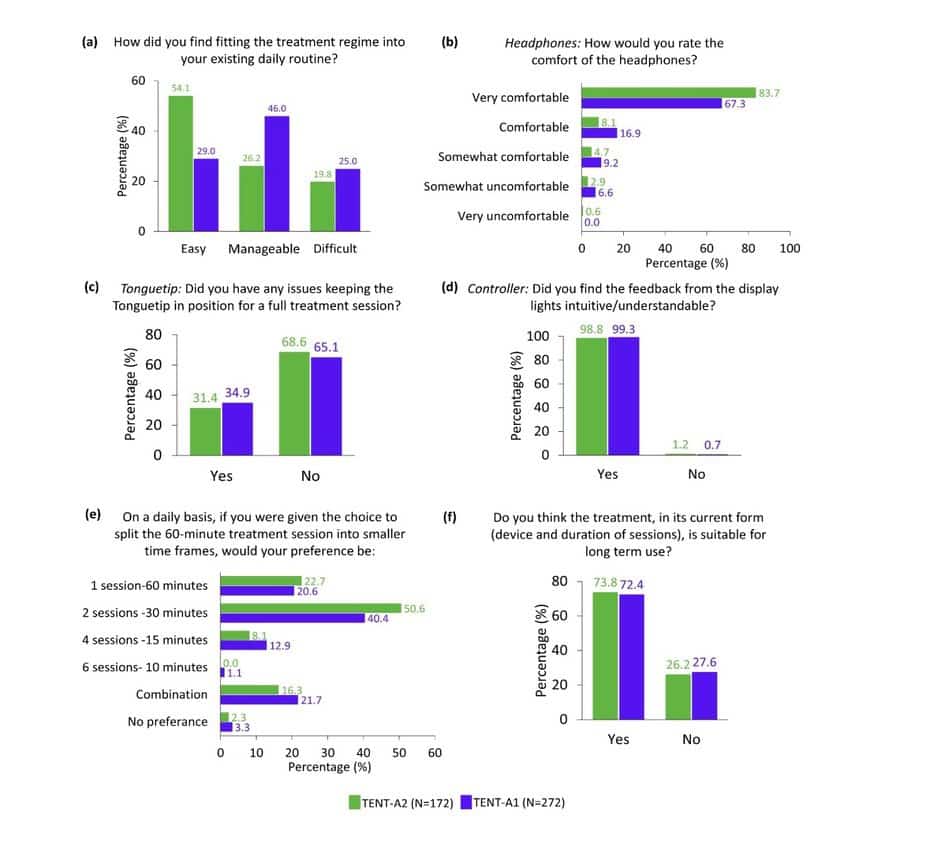
In terms of the feasibility of the Lenire treatment regime, there was a larger percentage of participants in TENT-A2 (54.1%) compared to TENT-A1 (29.0%) who responded that they found fitting the treatment regime into their existing daily routine easy (Figure 1a). In addition, 26.2% and 46.0% of TENT-A2 and TENT-A1 participants, respectively, responded that the treatment regime was ‘manageable’ to fit into their existing daily routine. There were comparable highly positive ratings in TENT-A2 and TENT-A1 in terms of the practicality of the headphones (Figure 1b), Tonguetip (Figure 1c), and Controller (Figure 1d). Also, in both studies, a clear majority of participants preferred the current recommended number of treatment sessions (Figure 1e), with over 70% of participants in both studies reporting that they think the current treatment is suitable for long-term use (Figure 1f). These encouraging repeatable results further build Neuromod’s confidence in the merit of the utility and usability of the Lenire treatment device and the generalizability of tinnitus treatment with Lenire.
Positive treatment effects in various aspects of life
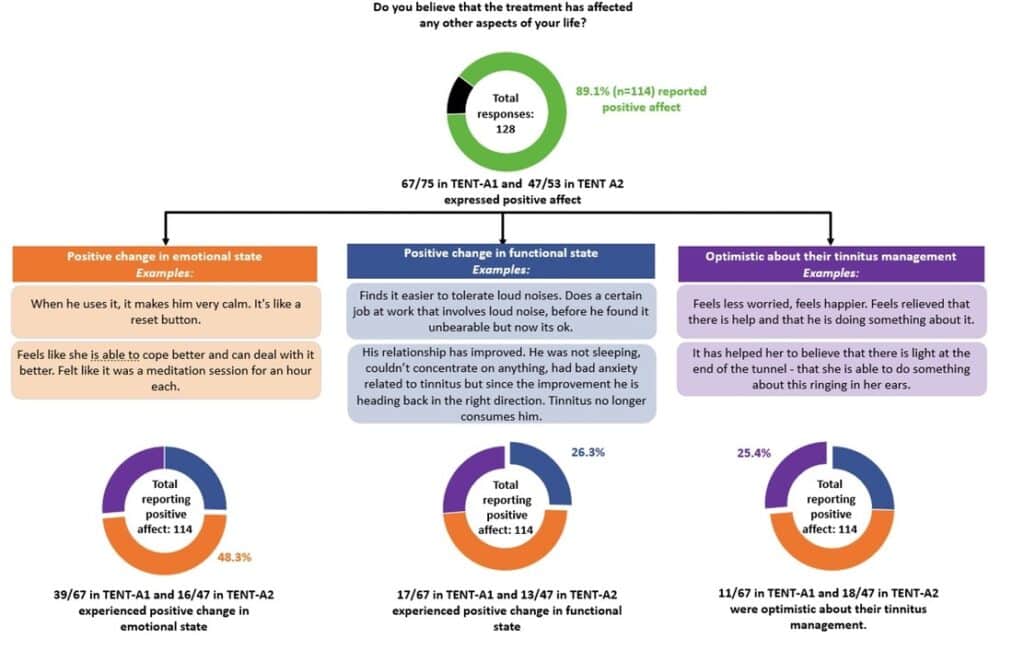
While structured assessments during exit interviews are important for guided exploration of participants’ experiences with the investigational treatment device, open-ended questions may provide an opportunity to capture important participant-perceived effects that would have otherwise been overlooked. During the TENT-A1 and TENT-A2 exit interviews, to gain a deeper appreciation of the effects of the Lenire treatment regime on daily life, participants were asked the exploratory question: ‘Do you believe that the treatment has affected any other aspects of your life?’
In TENT-A1 and TENT-A2, a subgroup of 128 participants who attended the exit interview responded to the open-ended question (Figure 2). In this analysis, responses were prioritized into one of the three identified themes. Of the 114 participants in TENT-A1 and TENT-A2 who expressed positive effects, 25.4% felt more optimistic about their tinnitus management (the remaining 74.6% of respondents did not provide additional detail), 48.3% had positive changes in an emotional state, and 26.3% reported positive changes in their functional state.
Tinnitus patients often report feeling distressed by their symptoms, with the American Tinnitus Association estimating that 75% of severe tinnitus patients suffer from stress, anxiety, and depression. The constant ringing also interferes with the patient’s ability to function normally. Improvement in mood and an increased ability to socialize and function support observations from other studies that these associated outcomes are important to patients with tinnitus. Also, given the limited treatment options that are consistently effective or accessible for the majority, it is heartening to know that the Lenire treatment offered hope and optimism to tinnitus sufferers regarding their tinnitus management.
Conclusion
In summary, exit interview results from TENT-A2 confirmed TENT-A1 findings that the portable Lenire device is comfortable to use and highly practical with a feasible treatment regime. In TENT-A1 and TENT-A2, further exploration of a subgroup of participants’ experiences and perspectives of the treatment provided insight into how using Lenire had affected relevant and meaningful aspects of their lives. Aside from the majority of participants reporting that they benefited from using Lenire, we learned that participants experienced positive changes in their emotional and functional states. Lenire also helped participants to feel more confident and optimistic about their tinnitus management. This collective evidence across two large-scale studies supports that the Lenire therapy represents great promise for hearing care professionals to successfully treat tinnitus patients.

Citation for this article: Ramirez T, Hamilton C, Leong SL. Bimodal Neuromodulation Treatment for Tinnitus Positively Affects Various Aspects of Life. Hearing Review. 2023;30(2):18-20
References
- Ramirez T, Hamilton C, Leong SL. Usability of Bimodal Neuromodulation to Treat Tinnitus. Hearing Review. May 2022.
- Conlon B, Langguth B, Hamilton C, et al. Bimodal neuromodulation combining sound and tongue stimulation reduces tinnitus symptoms in a large randomized clinical study. Science Translational Medicine. 2020;12(564).
- Conlon B, Hamilton C, Meade E, et al. Different bimodal neuromodulation settings reduce tinnitus symptoms in a large randomized trial. Scientific Reports. 2022;12(1):1-18.