Tech Topic | February 2022 Hearing Review
“Smaller, smarter medical devices are on their way, enabling a reimagining of the status quo.”
By David Akbari, AuD
Now that the FDA’s momentous final rule on over-the-counter (OTC) hearing aids has been finalized, many hearing healthcare professionals have lingering questions about the future of hearing products and services. This article will provide clarity on the final OTC rule and the impact that it will have now and in the future. To accomplish this goal, it is helpful to consider what the workforce will look like for people capable of servicing products in the market and look broadly at opportunities for technological advancement as it relates to over-the-counter hearing aids.
Since time immemorial, humans have been using some semblance of hearing assistance to help themselves communicate and connect with their environment. In the modern era, hearing healthcare professionals have worked together with those in need to overcome everything from various pathological and otologic conditions to being able to hear favorite television programs with greater clarity. Hearing aids broadly serve as an important lens through which audiologists and hearing instrument specialists are able to focus their skill sets. For this reason, it is worth reiterating that nothing can replace the high-touch, hands-on service delivery of a skilled licensed professional. However, over-the-counter hearing aids will touch the lives of many and bring the connection to tens of millions of un- and under-served Americans suffering from perceived mild-to-moderate hearing loss, who would otherwise have limited options for relief.
With this momentous rule increasing accessibility to the public, many are wondering how new over-the-counter products will differ from those products currently on offer via licensed professionals. The short answer to this inquiry is that the earliest hearing aid products themselves sold over the counter will be substantively similar to those already available by a licensed professional, though this will likely change in the years to come. While the OTC products will start out looking very similar to those which previously came via a licensed professional, the future of over-the-counter hearing aids will challenge our every notion of how a hearing aid looks and functions, to the point that they may be unrecognizable from what we imagine today.
The first differentiation to come will be to what extent there is self-fitting or other customization present as a method of programming settings since there will be no licensed professional involved. One of the most frequent questions surrounding the implementation of OTC hearing aids is, “is it possible to have an OTC hearing aid without self-fitting?” The answer to this is yes. As the FDA themselves describes in the final OTC rule.1
“Should a manufacturer incorporate self-fitting (or other) technology into one of its existing legacy or wireless devices and need to submit a 510(k), we would expect that the manufacturer could leverage the similarity with exempt devices as a least-burdensome way to obtain marketing authorization for the device that is not exempt from premarket notification requirements. Manufacturers of existing devices may not need to re-address questions, for example, related to electromagnetic compatibility (EMC), provided the manufacturer has not made changes that would affect EMC and require a 510(k) under our usual policies.”
In light of the FDA’s clarifying remarks noted here, it is no surprise that some manufacturers are leveraging their existing product portfolios into the over-the-counter market segment with the addition of self-fitting or other non-self-fitting customization potential. While it will be possible for the “big 5” to leverage their existing product portfolio to sell products into the over-the-counter market, it is unlikely they will actually do so. Doing so would risk cannibalizing the customer base of the “big 5” (namely, licensed professionals) by offering the same products without a prescription directly to the consumer. This need for differentiation opens the door to new market entrants and innovation in a way that has never in the history of hearing aids been observed before and will fundamentally change the definition of hearing healthcare in the United States and eventually around the world. Consumers will begin to take ownership of their condition and learn more about hearing, which will, in turn, develop and enhance the public’s perception of the value of hearing as a sensory modality. This demand for innovation will create exciting developments such as new test methods involving previously thought unimaginable ways to fit hearing aids that, for example, are not predicated on the results of a pure tone audiogram and instead on more practical top-down methods like a simple speech detection test as a method to derive hearing aid settings.
Now, more than ever in the history of hearing aids, it is imperative that audiologists seize the new opportunities afforded to them by the final over-the-counter hearing aid rule to differentiate themselves from mere device salespeople. A recent article by Bray and Amlani2 goes in detail through a workforce growth model for audiologists and hearing instrument specialists. This model expands and refines the earlier work done by Windmill and Freeman in 2013.3 The idea in both papers is that the increase in population numbers of people who could benefit from hearing aids will drive demand far greater than the profession could possibly service – even if all licensed professional dispensed hearing aids full time. While increasing demand is a key driver of the need for the over-the-counter hearing aid regulation, as Bray and Amlani2 point out, “the future success and viability of our profession and our scope will hinge on whether we can grow our workforce to match accelerating population demands, particularly between now and 2030.” Ergo, now is the time to reimagine how to best service the needs of the hearing impaired. To accomplish this goal, hearing professionals will need to scale the treatment model to grow beyond the provision of the device and focus on driving improved health literacy and public health outcomes as a key outcome of a successful treatment approach.
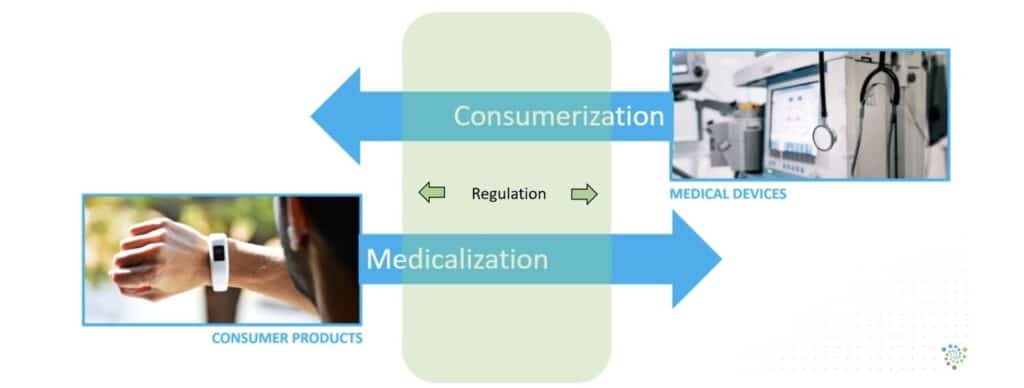
The best way to predict the future is to create it. With increasing innovation and consumer product choice, there will be an inevitable convergence of medical devices and consumer technology. Recently, consumers have flocked to the internet for information on over-the-counter hearing aids. There has been no shortage of product “top 10” lists and other rank ordering for consumers to inform themselves. While this underscores the importance of point-of-sale and consumer comparison as key benchmarks for the success of OTC regulation, there is a broader point to be made. The convergence of medical devices and consumer technology is a more generalized phenomenon, and there is an increasing amount of regulation that happens in the overlapping space between them Figure 1. Over-the-counter hearing aids happen to be a prime example of this phenomenon.
The devices of the future will ultimately be smaller, smarter medical devices featuring advanced biosensor integration intended for the treatment of hearing loss. All of this will be possible through the expanding regulatory framework enabling new market entrants, increased competition, and additional product offerings while ensuring high-quality standards are met, increasing public confidence. Americans will benefit from the increases in innovation, consumer choice, and affordability as companies strive to make better products and providers strive to meet the hearing-impaired consumer’s needs with greater success.
Notably, simply providing a consumer, especially one with no prior experience, with a hearing aid will not on its own accomplish the optimum treatment. The over-the-counter hearing aids of the future will have to focus on much more than the device and will drive engagement between the consumer and the licensed professional as the consumer’s understanding and care needs develop. The ecosystem of care model where there is long-term support after the product sale, an effective on-ramp to prescription hearing aids as they are needed, as well as advanced innovations in the aural rehabilitation space will also improve the public health outcomes as a direct result of the over-the-counter hearing aid regulation. Public health outcomes are the ultimate goal for over-the-counter hearing aids, not the sale of the device.
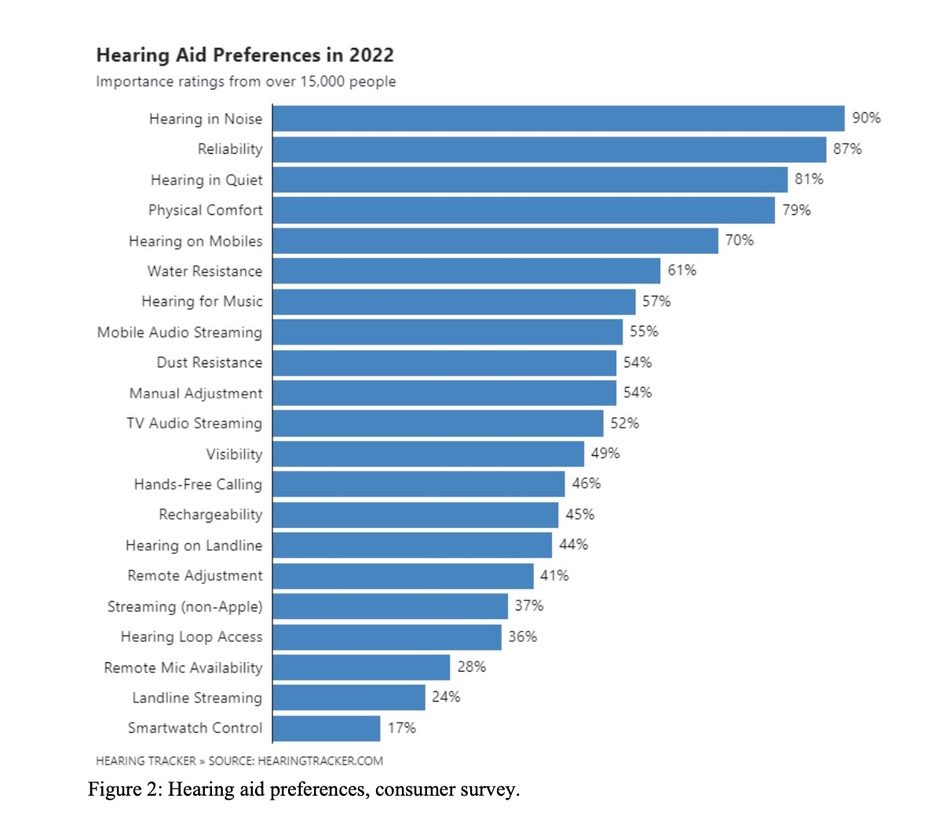
Opponents of over-the-counter hearing aids voice concerns broadly about under-amplification or a mismatch in self-selection leading to the providers’ perceived negative health consequences due to auditory cognition and neural plasticity changes. Contrary to widespread belief, this problem is not new to hearing aids, and certainly not new in the post-OTC landscape. For many years, the main reason people seek hearing aids (of any type) is also the main reason they are dissatisfied, hearing in background noise. Survey after survey confirms this fact, with a recent entry documenting Hearing Aid Preferences in 2022,5 Figure 2.
The main problem with conventional models of hearing aid selection and dispensing is that when you consider the use of the hearing aid as a medical device, the goal is to broadly manage loudness growth in the impaired ear (loudness being the perceptual correlate of intensity). Inappropriately, the profession’s solution is now, and has historically always been to make everything louder, _including_ the background noise! The problem with this approach is that when you consider the impaired ear, there are changes to the peripheral structures that lead to differences in inputs to the central auditory system. This results in changes in inputs to the brain where the brain reorganizes itself in response to this new louder stimuli but can never accurately reproduce the response that was present in the previously unimpaired ear. Thus, even in the best case, the traditional treatment model is asking hearing aid users to re-learn how to hear anyway because of fundamental physics limitations to this approach. Over-the-counter hearing aids will create innovation, such as reimagining the utility of pure tone audiometry and its role in hearing aid use. For example, new innovations in self-fitting using alternative stimuli could help bridge this gap and are only possible due to the FDA’s work in creating a foundational template where the spirit of American innovation can be unleashed.
With new standardized technologies rapidly emerging on the horizon, such as Bluetooth® LE, Auracast™, and the European Union’s interest in developing and promulgating their own version of over-the-counter hearing aids, there has never before been a more exciting time to be involved in reimagining the future of hearing healthcare. Though there may be ideological differences in perception among how OTC hearing aids will impact the public, the free market and retail points of sale will have a cross-synergistic effect to know what works and what does not among consumers in the market. Successes in OTC hearing aids can help inform and improve best practice models for prescription hearing healthcare and vice-versa, with the utopian ideal that public health outcomes can improve in ways not previously thought possible and enabled by the final OTC hearing aid rule.
The year 2023 promises to bring even more products into the market and, crucially, more options beyond devices to improve and enhance hearing healthcare, heralding a new golden age of consumer choice and provider advocacy. When the least privileged among us can benefit from unprecedented and emerging levels of access and affordability thanks to the now final OTC hearing aid regulation, all Americans benefit. The future promises to bring an exciting era of smaller, smarter biosensor-enabled medical devices that can help improve people’s quality of life, reduce social isolation and cognitive decline, and increase public awareness and knowledge of hearing loss. When people work together to reimagine what is possible for the benefit of all, everyone wins, and the future is that much brighter.

Citation for this article: Akbari D. OTC hearing aid rule – the impact now and into the future. Hearing Review. 2023;30(2):8-11.
References:
1. Federal Register. Medical devices; ear, nose, and throat devices; establishing over-the-counter hearing aids. https://www.federalregister.gov/documents/2022/08/17/2022-17230/medical-devices-ear-nose-and-throat-devices-establishing-over-the-counter-hearing-aids. Published August 2022.
2. Bray V, Amlani A. Audiology workforce: a new analysis of the audiology workforce, benchmarked to other healthcare professions. Audiology Practices. 2022;14(4): 44-54.
3. Windmill I, Freeman B. Demand for audiology services: 30-yr projections and impact on academic programs. Journal of the American Academy of Audiology. 2013 May;24(5):407-416.
4. Ko A. Developing the ultimate medical sensor technology. Medical Design Briefs. https://www.medicaldesignbriefs.com/component/content/article/tb/webcasts/upcoming-webinars/46873.
5. Bailey A. The Ultimate Guide: Types, Features, Prices, Reviews, and More. https://www.hearingtracker.com/hearing-aids. Published October 2022.






What guarantee can the manufacturers provide to the patient (the consumer), that further progression of SNHL. will not take place and the the cases of dementias will begin to drop?
Any challengers please?
Until this is dealt with, I only see an explosion of deciet and fraud in this corrupt industry. Sad but true!
Anjan Muhury
Investigator, Hearing Loss SNHL.