The US Food and Drug Administration (FDA) has cleared MED-EL’s PULSAR, SONATA, and MED-EL CONCERT cochlear implants for use with 1.5 Tesla (T) MRI systems without the need for surgical removal of the internal magnet.
Patients with one of these MED-EL cochlear implants will be able to obtain 1.5T MRI scans upon final labeling approval by the FDA, expected by the end of summer 2013. Earlier in 2013, MED-EL’s Sophono Alpha 2 Hearing System had been cleared by the FDA for 1.5T.
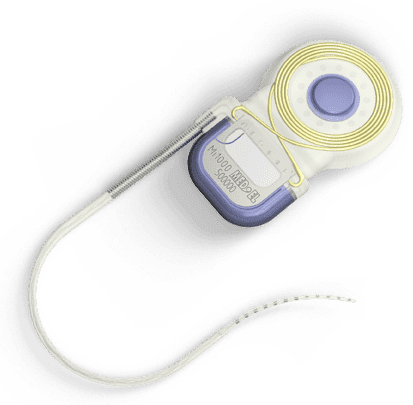
|
| The MED-EL CONCERT cochlear implant is among the models now approved to be used with a 1.5T MRI system. |
Currently, these MED-EL cochlear implants are the only implants available in the United States that can be used with a 1.5T MRI, according to the company. MRI machines are available in a range of strengths. The current standard strength is 1.5T in the United States, and the vast majority of MRIs performed are done with 1.5T machines.
Magnetic resonance imaging capability can be an important issue for people who have cochlear implants. The FDA approval for MRI imaging at 1.5T without the surgical removal of the magnet is helpful for people who live with chronic conditions, such as heart disease, cancer, and neuromas, that may require the regular use of MRI technology.
All MED-EL cochlear implants have been FDA approved for 0.2T scans without surgical removal of the magnet since 2001. The addition of 1.5T MRI means that physicians will be able to obtain even higher resolution imaging, allowing better diagnosis and treatment using the most popular scan resolution currently used in the United States.
Pete Unger, a member of MED-EL’s Patient Support Team, is one such case. After he had surgery to remove a tumor in 2004, Unger’s doctors recommended periodic MRIs to monitor the area where his tumor was removed. The surgery left Unger completely deaf on his right side and he soon lost most of his hearing on his left side due to Meniere’s disease. Unger says that MRI capability without magnet removal was a primary reason why he chose a MED-EL implant in 2007.
“It’s reassuring to know that my MED-EL implant has not only given me my hearing back, but has now given me peace of mind during my follow-up care,” he added.
Patients with other brands of cochlear implants must have minimally invasive surgery to remove the internal magnet before undergoing an MRI scan and a second surgery is required to replace the magnet after the scan has been obtained.
People who have a MED-EL PULSAR, SONATA, or MED-EL CONCERT cochlear implant will not need special permission from the company prior to undergoing an 1.5T MRI; warranties for internal equipment will continue to be valid as long as the company’s recommendations for scanning are followed. These recommendations will be available on the company’s website in the near future. It is important to note that all cochlear implants, including those manufactured by MED-EL, remain contraindicated for MRIs at 3.0T strength in the United States.
SOURCE: MED-EL





