Should HCPs monitor patients who have contracted COVID-19 for exacerbated hearing loss? A new study investigates this association between the virus and hearing.
By Jonathan Mikhail, EdD, AuD, MS; and Arsenio Paez, PhD
Because it is common for viral infections to cause hearing problems, audiologists and otologists often encounter patients with resulting hearing loss. However, little is known about hearing loss following COVID-19 infection, making it difficult for hearing care professionals to know how to address possible resulting effects in clients.
Early studies in this area focused on asymptomatic patients who had contracted the virus and had no pre-existing hearing loss or other medical issues related to auditory diseases.(1) For example, case studies have noted the possibility of sudden onset sensorineural hearing loss,(2,3) and some case studies using post-contrast MRI found inflammation of the cochlea after contracting COVID-19.(4) Because of these findings and other considerations, some investigators have called the issue of hearing loss after contracting COVID-19 a widespread problem that should be addressed in patients with otological and audiological disorders.(5)
Other auditory disorders have also been associated with COVID-19. A case study described the effect of the virus on a patient’s balance, causing vestibular neuritis or labyrinthitis.(6) Tinnitus is another reported symptom following the virus in patients who did not previously have tinnitus.(7) However, to our knowledge, no observational study has investigated the association between pre-existing hearing loss and exacerbated hearing loss following COVID-19.
Exacerbated hearing loss after COVID-19 infection can be combined with other factors related to the virus and pandemic, creating increased difficulties for people affected by the virus. For example, personal protection devices such as plexiglass, masks, and other face coverings have been used throughout the pandemic. While protecting people, these devices can interfere with lipreading, a valuable tool used by people with pre-existing hearing loss, which affects their perception of auditory stimuli.(2) Other published articles have emphasized how many patients struggle with hearing loss and how these barriers cause incredible difficulty, especially when additional hearing loss occurs, and even more so in background noise and other demanding communicative situations.(8) Social distancing has also played a role in diminished speech intelligibility, with many individuals remaining six feet apart in certain situations.
A greater understanding of the range of potential symptoms associated with the virus is necessary to pinpoint what may or may not be associated with COVID-19.(9) Hearing loss has long been a public health concern. This study aimed to evaluate a sample of patients who had contracted COVID-19, ruling out any potential association with presbycusis or other comorbidities, to assess possible damage due to the virus. Secondly, this study set out to evaluate whether word recognition scores and speech reception thresholds were also affected by the spread of the virus. Lastly, this study assessed the possibility that sex differences in COVID-19 exacerbated hearing loss.
Methods and Materials
In this observational study, a case history questionnaire was administered to potential participants before the testing began. The questionnaire ruled out any additional pathologies that could affect the peripheral hearing system, including otitis media, vestibular schwannoma, or medication changes that could cause hearing loss. The case history also considered the patient’s candidacy based on the participant’s time of contracting COVID-19. To participate, participants had to contract COVID-19 within six months of March 15, 2020.
Potential participants had to have been diagnosed with bilateral symmetrical sensorineural hearing loss by an audiologist within one year before contracting COVID-19. Before participating in the study, participants had to have all testing materials, including air and bone conduction, word recognition scores, and speech reception thresholds, identified using the CPT code 92557. Participants also had to provide documentation of a confirmed positive COVID-19 test performed at a pharmacy, hospital, or county health department with quarantine orders. Individuals who qualified for the study had to contract the virus before Sept. 15, 2020.
Recruitment and Sample Overview
One clinic, Area Hearing & Speech Clinic Inc. in Joplin, Missouri., was used for the data collection process. The clinic’s database was used with permission from the clinic owner to determine who of the clinic’s patients met the age requirement (50-64); had bilateral, symmetrical sensorineural hearing loss; and had had a full audiological assessment that matched the procedure CPT code 92557. If an individual met the age and hearing loss requirements, the original questionnaire was distributed to inquire about COVID-19 contraction, quarantine orders, and any contraindications for participation in the study.
The target population for this study was participants aged 50-64 years, with the premise that presbycusis would not be a significant factor in this age group. All participants were diagnosed with bilateral symmetrical sensorineural hearing loss before COVID-19. Thirty participants (18 females and 12 males) participated in the study. The mean age of participants was 57.47.
The same GSI Audiostar audiometer was used for consistency during the experimental phase. Calibration occurred one month before data collection, ruling out variability resulting from calibration errors. Each participant was tested using the same testing sound booth. RadioEar IP30 insert earphones with E-A-RLink Adult Eartips headphones were used by all the participants. For each participant, a one-quarter mono-plug patient response button was attached to the audiometer. The RadioEar B71 Bone Conduction Headband was also used for bone conduction testing.
An independent audiologist performed a secondary audiological assessment of eligible participants to avoid tester bias. The testing was distributed as follows: air conduction, speech reception thresholds (recorded spondees, list A), word recognition testing (NU-6, lists one and two), and bone conduction. If cerumen management was required, this occurred before testing began by using a curette. Irrigation cerumen removal and suction were not permitted due to fear of temporary threshold shifts.
A copy of the original audiometric assessment, including the SRTs and WRSs for each ear, was inputted into the HIMSA NOAH Audiogram module. The independent audiologist entered all new audiometric data into the same HIMSA NOAH software for each participant. Once data were acquired from all 30 participants and entered into the HIMSA NOAH Audiogram Module, they were analyzed using the Statistical Package for the Social Sciences (SPSS).(10)

Figure 1. PTAs for the right left ear, pre- and post-COVID-19 infection
Data Analyses
The Kolmogorov-Smirnov (K-S) and Shapiro-Wilks (S-W) tests indicated a normal distribution of the participants’ characteristics. Using a three-frequency puretone average (PTA), including 500 Hz, 1000 Hz, and 2000 Hz, each participant’s results were evaluated for shifts within the PTA before and after post-COVID-19 contraction. This was performed between each ear from the first to the second audiological assessment.
A paired samples t-test showed that the participants’ level of hearing loss increased from pre-COVID (M = 31.43, SD = 9.47) to post-COVID-19 contraction in the right ear (M = 38.59, SD = 12.86; t = -4.1, p < .001). The same test was performed in the left ear, which also exhibited increased hearing loss pre- (M = 32.35, SD = 8.16) and post-COVID-19 (M = 39.14, SD = 13.76; t = -3.59, p < .001).
Further reading: COVID-19 Virus May Increase Risk of Neurological Conditions
WRS and SRT Results
A paired samples t-test in the right ear indicated that the participants’ WRSs did not change significantly from pre-COVID (M = .93, SD = .05) to post-COVID-19 contraction (M = .88, SD = .11; t = 2.0, p = .290). The same test was performed in the left ear, also indicating no regression in WRSs pre- (M = .91, SD = .08) and post-COVID-19 infection (M = .86, SD = .13; t = 2.05, p = .0.98).
A paired sample t-test in the right ear indicated that the participants’ SRTs did exhibit a significant change from pre-COVID (M = 30.83, SD = .08) to post-COVID-19 contraction (M = 36.00, SD = 13.6; t = -2.46, p = .002). The same test was performed in the left ear, which also indicated significant changes in SRTs pre- (M = 32.50, SD = 8.88) and post-COVID-19 (M = 39.67, SD = 16.5; t = -3.00, p < .001).
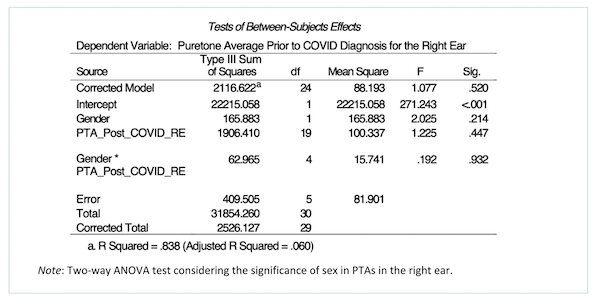
Table 1.PTA-sex comparison, right ear.
Sex Comparison
Sex was an additional variable explored in the association between COVID-19 and additional hearing loss in this study. A two-way ANOVA test was performed to analyze the effect between males and females on hearing loss regression post-COVID-19 contraction in the right and left ear. The results in the right ear indicated no significant main effect for sex, F (1, 5) = 2.02, p = .214; no significant main effect for PTA regression post-COVID-19 infection, F (19, 5) = 1.23, p = .447; and no significant interaction between gender and PTAs post-COVID-19 infection, F (4, 5) = .192, p = .932. The results in the left ear indicated no significant main effect for sex, F (1, 8) = .224, p = .648; no significant main effect for PTA regression post-COVID-19 infection, F (18, 8) = 2.30, p= .116; and no significant interaction between sex and PTAs post-COVID-19 infection, F (2, 8) = .359, p = .709.
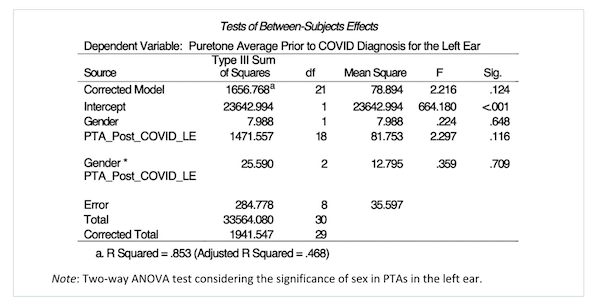
Table 2.PTA-sex comparison, left ear.
Discussion
This study investigated the association between COVID-19 and pre-existing hearing loss. Our study found that COVID-19 was associated with increased (exacerbated) hearing loss in individuals with hearing loss existing before contracting the virus. Although word recognition scores (WRS) did not show a significant change if compensatory volume was given to patients who had lost more hearing, their speech reception thresholds (SRTs) regressed. This is expected, as peripheral hearing decreases since SRTs are the basic speech threshold that quantifies an individual’s hearing threshold level for speech.(11)
While more research is needed to understand how COVID-19 affects the auditory system, the results of this study suggest that individuals with pre-existing hearing loss require further audiological monitoring after contracting COVID-19. This may be of particular importance for audiologists in clinical practice with individuals at heightened risk of COVID-19 infection or hearing loss.
Other hearing disorders have been associated with both COVID-19 and hearing loss. For example, tinnitus is a well-documented side effect of COVID-19 and can be accompanied by hearing loss.(12,7,13) In individuals with pre-existing tinnitus, COVID-19 infection may also be a risk factor for accelerated hearing loss, which could be considered when monitoring their hearing or evaluating them for tinnitus intervention. While this study did not focus on tinnitus as the underlying cause of hearing loss, our results may support additional audiological testing or monitoring after COVID-19 infection. Researchers may also establish a testing protocol to monitor subjective and measured tinnitus in persons who have contracted COVID-19, to evaluate whether accelerated hearing loss is associated with COVID-19 in persons with tinnitus.
Recommendations
Future studies with larger sample sizes and larger age ranges are needed to explore the association between increased hearing loss and COVID-19. COVID-19 may be associated with increased hearing loss in younger or older adults with already diagnosed hearing disorders. Additionally, while we found no statistically significant association between sex and increased hearing loss experienced by participants in this study, it is possible that a larger study may find gender-based differences that we were underpowered to detect.
This type of research should be considered for pediatric patients with hearing loss as well. Although younger persons with COVID-19 tend to have fewer significant symptoms and shorter recovery times than adults due to a lack of other pre-existing conditions, this population should also be examined for further hearing loss.(14) Such studies can improve our understanding of how COVID-19 can cause additional hearing loss in persons with prediagnosed hearing loss before contracting the virus. Our protocol can be adapted for routine assessment in children. For children who do not qualify for voluntary testing, auditory brainstem response and condition play audiometry could be facilitated to monitor hearing loss after COVID-19.
The third area of potential research involves older adults. Our study excluded participants older than 64 years to reduce the confounding effects of presbycusis. Given that older adults (greater than 64 years) are at a heightened risk of COVID-19 and health complications, further research should be conducted to consider the interaction between COVID-19 and presbycusis.(15-16) Older adults may often experience hearing loss regardless of COVID-19 infection, and this must be considered in future studies.
An additional avenue for future research is the effect of COVID-19 on hearing loss in individuals who have contracted the virus multiple times. Although many people contracted the virus during the first wave of the pandemic, studies could explore whether different strains of the COVID-19 virus or repeated exposure to them had differential effects on the rate of accelerated hearing loss.
Conclusion
In this observational study, we investigated the association between COVID-19 and pre-existing hearing loss. We found an association between COVID-19 infection and the acceleration of symptoms in individuals with pre-existing hearing. Otologists, audiologists, and primary care physicians should consider the potential impact of COVID-19 in patients with pre-existing hearing loss and encourage patients to seek audiological evaluation of the potential for additional hearing loss. Further research on the effects of COVID-19 on the auditory system can provide valuable insights into how COVID-19 and other viruses affect hearing and guide clinical practice guidelines for audiological testing in vulnerable populations.
Jonathan Mikhail, EdD, AuD, MS, is an audiologist and health scientist at the Area Hearing and Speech Clinic in Joplin, Missouri, and adjunct faculty at Wichita State University in the Doctor of Audiology program. He has presented extensively on COVID-19, hearing loss, audiology education, auditory processing disorders, and private practice management. He is also the director of the audiology residency program at the clinic where he works.
Arsenio Paez, PhD, is a clinical trials methodologist focusing on complex interventions, including sleep, surgically implanted medical devices, and CNV genetic disorders. His research interests include circadian neuroscience, sleep, and lifestyle factors such as physical activity on physical and mental health. He is with the University of Oxford, UK, Northeastern University, School of Clinical and Rehabilitation Sciences, and the Doctor of Education in Health Professions Program at A.T. Still University.
Original citation for this article: Mikhail J., Paez A. COVID-19’s Effect on Pre-existing Hearing Loss. Hearing Review. 2024;31(4):20-23.
References
- Mustafa MWM. Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am J Otolaryngol. 2020;41(3):102483. doi:10.1016/j.amjoto.2020.102483
- Reed, N. S., Ferrante, L. E., & Oh, E. S. (2020, July 2). Addressing hearing loss to improve communication during the COVID‐19 pandemic. Journal of the American Geriatrics Society, 68(9), 1924–1926. https://doi.org/10.1111/jgs.16674
- Koumpa FS, Forde CT, Manjaly JG. Sudden irreversible hearing loss post COVID-19. BMJ Case Rep. 2020;13(11):e238419. Published 2020 Oct 13. doi:10.1136/bcr-2020-238419
- Degen C, Lenarz T, Willenborg K. Acute Profound Sensorineural Hearing Loss After COVID-19 Pneumonia. Mayo Clin Proc. 2020;95(8):1801-1803. doi:10.1016/j.mayocp.2020.05.034
- Fidan V, Akin O, Koyuncu H. Rised sudden sensorineural hearing loss during COVID-19 widespread. Am J Otolaryngol. 2021;42(5):102996. doi:10.1016/j.amjoto.2021.102996
- Karimi-Galougahi M, Naeini AS, Raad N, Mikaniki N, Ghorbani J. Vertigo and hearing loss during the COVID-19 pandemic – is there an association?. Acta Otorhinolaryngol Ital. 2020;40(6):463-465. doi:10.14639/0392-100X-N0820
- Jafari Z, Kolb BE, Mohajerani MH. Hearing Loss, Tinnitus, and Dizziness in COVID-19: A Systematic Review and Meta-Analysis. Can J Neurol Sci. 2022;49(2):184-195. doi:10.1017/cjn.2021.63
- Ten Hulzen RD, Fabry DA. Impact of Hearing Loss and Universal Face Masking in the COVID-19 Era. Mayo Clin Proc. 2020;95(10):2069-2072. doi:10.1016/j.mayocp.2020.07.027
- Wagatsuma Y, Daimaru K, Deng S, Chen JY. Hearing loss and the COVID-19 pandemic. BMC Res Notes. 2022;15(1):228. Published 2022 Jun 27. doi:10.1186/s13104-022-06120-1
- IBM Corp. Released 2020. IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp
- American Speech-Language-Hearing Association. (n.d.). Determining threshold level for speech. https://www.asha.org/policy/gl1988-00008/#:~:text=The%20basic%20purpose%20of%20a,for%20the%20pure%20tone%20audiogram.
- Beukes EW, Baguley DM, Jacquemin L, et al. Changes in Tinnitus Experiences During the COVID-19 Pandemic. Front Public Health. 2020;8:592878. Published 2020 Nov 5. doi:10.3389/fpubh.2020.592878
- Viola P, Ralli M, Pisani D, et al. Tinnitus and equilibrium disorders in COVID-19 patients: preliminary results. Eur Arch Otorhinolaryngol. 2021;278(10):3725-3730. doi:10.1007/s00405-020-06440-7
- Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID-19: Why Children Fare Better than Adults?. Indian J Pediatr. 2020;87(7):537-546. doi:10.1007/s12098-020-03322-y
- Singhal S, Kumar P, Singh S, Saha S, Dey AB. Clinical features and outcomes of COVID-19 in older adults: a systematic review and meta-analysis. BMC Geriatr. 2021;21(1):321. Published 2021 May 19. doi:10.1186/s12877-021-02261-3
- Morrow-Howell N, Galucia N, Swinford E. Recovering from the COVID-19 Pandemic: A Focus on Older Adults. J Aging Soc Policy. 2020;32(4-5):526-535. doi:10.1080/08959420.2020.1759758