This study investigates the effects of contralateral masking noise on the click-evoked Pa waveform of the human auditory middle latency response (AMLR). Normative values for latencies and amplitudes of the Pa waveform for adults along with confidence intervals are also provided.
During clinical diagnostic testing, such as in the presence of auditory nerve tumor on one side, the type (sensorineural versus conductive) of hearing loss on the pathological side can be accurately determined by applying masking noise to the normal contralateral ear. Such contralateral noise suppresses the response of the normal ear to test stimuli that are presented to the ear with pathology, and allows fairly accurate determination of the severity and type (conductive versus sensorineural) of hearing loss in the pathological ear.
AMLR and Contralateral Noise
Potential effects of contralateral noise on the ipsilateral response. Application of noise to the contralateral ear can somewhat affect responses of the ipsilateral ear, even though the contralateral noise is not high enough to cross over to the other side and stimulate the ipsilateral ear.
Wegel and Lane1 referred to this type of masking as central masking. Central masking probably results from binaural interactions that are possible at various levels of the central auditory system, including the inferior colliculus, medial geniculate bodies, and the corpus callosum. The investigations of the presence of such binaural interactions are important in understanding the function of the central auditory system. On the other hand, if a specific response is not significantly affected by masking noise presented to the contralateral ear, this can allow assessment of the function of each ear separately by masking the other ear.

|
Vishakha W. Rawool, PhD, is a professor in the Department of Speech Pathology and Audiology at West Virginia University, Morgantown, WVa, and Maria V. Brouse, AuD, is a clinical audiologist at Red Rose Hearing Center in Lancaster, Pa. |
The effects of contralateral noise on ipsilateral responses have been demonstrated in behavioral studies. On average, a decrease of 5 dB is apparent in psychoacoustic thresholds following the introduction of contralateral noise, but the shift varies considerably across individuals. Central masking is more noticeable when the contralateral masker and the ipsilateral signal are pulsed on and off together. Central masking is minimal (1 or 2 dB) regardless of the masker level when the signal is pulsed but the contralateral noise is continuous.2,3
Auditory middle latency response (AMLR). The AMLR is a far-field response that occurs between 10 and 90 ms following the onset of a transient auditory stimulus, and can be recorded by placing electrodes on the scalp. The typical AMLR in humans can be characterized by six components: three negative (No, Na, Nb) and three positive (Po, Pa, Pb).4
Of all these components, Pa is considered to be the most constant and reliable.5 Pa is also considered the most sensitive component for audiological assessments.6 In adults, the Pa component occurs approximately 20 to 40 ms following stimulus onset.7,8 With moderately high-intensity stimuli of 70 to 75 dB nHL, Pa appears as a symmetric positivity over the central (Cz) and frontal (Fz) regions of the adult human scalp.7,9,10 If the nasion is used as a reference electrode, Pa positivity appears to remain midline and symmetric regardless of the ear of stimulation.7
Generator sources for the Pa waveform. There is some controversy about the generator sources of Pa.11 Portions of the primary auditory pathway, including the temporal lobe and thalamus, may contribute to the generation of the Pa waveform.12 Topographic studies,13 magnetoencephalography source analyses,14 and cortical recordings15 also suggest that Pa is generated in the auditory cortex. However, Pa may also be influenced by some deep midline brainstem generators.10,16
Clinical Use of AMLR
The AMLR has been used in several clinical settings. AMLR appears to be a useful indicator of the hypnotic state during anesthesia17 even in the presence of hearing loss.18 The Pa component of the AMLR can serve as an indicator of implicit memory formation during general anesthesia.19 It can also be used for predicting the possibility of awakening in comatose patients.20
Children with learning disabilities yield asymmetric latencies for contralateral recordings of the AMLR. When the weaker ear is presented with a stimulus, processing within the contralateral pathway is delayed in these children.21 Pa response can also be used to assess hearing sensitivity for low frequency (pitch) stimuli.6,22-25 In addition, it is useful for neuroaudiologic evaluations.22,26 For example, Japaridze et al27 showed that the AMLR is useful in detecting and confirming multiple sclerosis.
AMLR has also been used for assessment of patients with tinnitus and hearing loss28 and for assessment of auditory processing disorders.29,30
Effects of contralateral noise on guinea pig AMLRs. Although no reports about the effect of contralateral noise on the human AMLR are available, some reports about the effect of contralateral noise on click-evoked temporal and midline AMLRs in guinea pigs have been published. The temporal AMLR is recorded by placing active electrodes on the temporal region, while the active electrode is placed on the posterior midline of the skull to obtain the midline AMLR.
Ozdamar et al31 reported an increase in temporal AMLR amplitudes with contralateral noise of 50 dB HL with click levels varying from 30 to 60 dB HL. They speculated that noise masking suppresses an existing inhibitory process. They further suggested that such inhibitory input probably comes from either the contralateral auditory cortex via the corpus callosum or from other subcortical centers.
Littman et al32 investigated the effects of contralateral noise of 70 dB peSPL (approximately 40 dB HL) on the temporal and midline AMLR evoked with clicks presented at 70 dB peSPL. Contralateral noise increased the latencies of the temporal AMLR and decreased the amplitudes for one of the wave components. For the midline AMLR, the noise caused a significant decrease in the amplitudes of all components and an increase in latency for one of the wave components. Littman et al suggested that the difference in their and the Ozdamar et al31 study might be due to the guinea pigs’ state of arousal: in the Ozdamar et al study, the animals were awake; in the Littman et al study, the animals were anesthetized.
It was suggested that anesthesia inhibits the mechanism that causes noise-induced enhancement of the amplitude of the temporal AMLR. Littman et al32 further speculated that the effect of contralateral noise is different on the temporal and midline AMLR because in guinea pigs the temporal AMLR is predominantly generated by the primary auditory cortex while the midline response is primarily generated in the association pathways.33
Goksoy and Utkucal34 investigated the effects of anesthesia on the temporal AMLR. They reported an enhancement of the guinea pig temporal AMLR due to contralateral noise (Click level: 55 dB HL; Noise level: 55 dB). The enhancement effect gradually decreased and disappeared with anesthesia, suggesting that higher neural regions are responsible for this effect, because higher centers are known to be more readily suppressed by anesthesia.
Goksoy et al35 reported a significant increase in the guinea pig temporal AMLR with continuous white noise up to 350 ms following the initiation of the noise. However, after 2 and 5 ms, the continuous white noise decreased the AMLR amplitudes. These investigators hypothesized that the short-term AMLR enhancement resulting from continuous white noise occurring within 350 ms is an on-response type effect that originates from low-level binaural centers such as the olivocochlear bundle. After 2 seconds, the same noise decreases the AMLR amplitudes due to contribution from high-level binaural centers such as subcortical inhibitory connections.
The above guinea pig studies collectively suggest that contralateral noise briefly enhances the temporal AMLR in awake guinea pigs, and with continuation of noise beyond 2 to 5 seconds, the AMLR amplitudes decrease. It should be mentioned that investigators have noted enhancement due to contralateral noise31,34 using high levels of noise, while Littman et al32 used somewhat lower noise levels and did not find the enhancement. It is possible that enhancement or decrease of the AMLR may be dependent on the level of the noise in addition to noise duration and subject alertness.
Rationale for the current investigation. Guinea pig AMLRs are not easily comparable to human AMLRs due to differences in waveform morphology and relative amplitudes at different recording locations. For example, human AMLRs are symmetrical around the vertex, but guinea pig AMLRs are lateralized to the contralateral side.32
To the authors’ knowledge, the effect of contralateral noise on the human AMLRs has not been reported so far. Thus, the goal of the current study was to investigate the effects of contralateral noise on human AMLRs.
Methods
Participants. A total of 30 women were included in the study. Gender can have a significant effect on the Pa waveform of the AMLR. Pa amplitudes tend to be larger and Pa latencies tend to be shorter in women.36 Therefore, only women were included in the study to provide a fairly large sample of participants with the same gender while controlling for gender-related variability. Participants ranged in age from 19 to 28, with an average age of 23 years. This age range was selected to ensure a similar range of normal hearing sensitivity and AMLR waveform morphology37 since age is known to have an effect on Pa amplitudes.38-40
Participants were selected on the basis of no known neurogenic abnormalities, normal otoscopic findings, normal auditory sensitivity, and normal tympanometric findings. Prior to testing, participants read and signed an informed-consent form approved by the Institutional Review Board. All data collection followed the ethical standards of the Helsinki Declaration.
Criteria for normal auditory sensitivity included pure-tone thresholds within 20 dB HL for octave intervals between 0.25 and 8 kHz. Tympanometric findings were considered normal based on the guidelines provided by ASHA: Compensated static admittance within 0.3 to 1.4 ml range, ear canal volume between 0.6 and 1.5 ml, tympanometric width between 50 and 110 daPa, and middle ear pressure between +100 and -100 daPa.41
Stimuli. A Nicolet Spirit Evoked Potential System (v1.70) was used for stimulus presentation. Auditory stimuli were presented through Nicolet Model Tip-300 insert earphones. Rarefaction clicks were presented at 80 dB nHL (approximately 115 dB peSPL) since Pa is more prevalent at such levels.42 Only rarefaction clicks were used to control the possibility of any variability due to click-polarity.43,44
Clicks were always presented to the left ear, as a significant ear effect on Pa latency has been noted by previous investigators.7,45 The presentation rate was 1.1 clicks/sec since slower click rates yield largest Pa amplitudes.36 Use of such a slow rate is also likely to reduce the potential effects of acoustic reflex, which can be elicited due to the use of high intensity clicks.46
AMLRs were obtained for four different stimulus conditions: clicks only to the left ear; clicks to the left ear along with 30, 40, and 50 dB nHL (approximately 60, 70, and 80 dBSPL) of continuous digitally generated broadband noise to the right ear.
Noise levels higher than 50 dB nHL were avoided since the interaural attenuation values for insert earphones vary from 60 to 100 dB.47 Thus, higher noise levels have the potential for reaching the test ear through acoustic crossover. Continuous noise was used since in clinical applications this type of noise is used to minimize the participation of the non-test ear.
Recording of responses. Neuro-electric activity was recorded by placing silver-silver electrodes on ipsilateral and contralateral earlobes and vertex, with ground on the low forehead. This electrode montage appears to be best suited for recording the Pa waveform of the AMLR9,42 and is also appropriate for use in clinical settings. Impedances at each electrode site were maintained at or below 5 KOhms.
Participants were seated in a reclining chair. They were instructed to sit back and focus on a picture on the wall in front of them. The importance of staying awake was emphasized. They were informed that, in the middle of the test, a break will be offered, if necessary. Participants were allowed to have a break after the first four recordings to ensure alertness. Although the electrodes were not detached from the scalp during the break, impedances were rechecked following any breaks.
Studies have shown that a band-pass setting with a high-pass filter of 1 Hz and a low-pass filter of 100 or 500 Hz yields robust easy-to-identify AMLR waveforms.48 A high-pass filter of 1 Hz and low-pass filter setting of 100 Hz (6 dB/octave slope) were used in the current study because pilot recordings using these filter settings yielded clearest Pa waveforms. Averaging occurred over a 100 ms time base.
Two-channel recordings consisting of ipsilateral and contralateral responses were obtained. Two separate recordings (second recording immediately following the first) consisting of 256 averaging sweeps were obtained for each test condition to ensure reliability of data. Thus, a total of 8 recordings (4 test conditions) were obtained from each participant. Test-condition order was random. Due to the 2-channel recording, this resulted in 16 AMLR traces for each participant. All data were stored on a computer disk for off-line analyses.
Pa waveform was identified because it is the most consistent and reliable component of the AMLR.42,48 Pa was considered to be the relatively broad positivity that occurred between 20 and 40 ms following stimulus onset.
Both investigators determined Pa latencies and amplitudes collaboratively. The amplitudes of the Pa waveform were measured as peak-to-peak amplitude from Pa to Nb to the nearest 0.01 µV. The latencies were measured to the nearest 0.01 ms.
Statistical analyses. Test-retest reliability of recordings across Trial 1 and Trial 2 was evaluated by submitting the latencies and amplitudes to the Pearson product-moment correlations procedure. In addition, the repeated Measures Multivariate Analyses of Variance (MANOVA) was used with three independent variables: 1) Test condition (clicks only, clicks plus 30 or 40 or 50 dB contralateral noise); 2) Recording mode (ipsi- and contralateral); and 3) Test trial (Trial 1 and 2). Latency and amplitude data (dependant variables) were analyzed with separate MANOVAs. Post-hoc analyses were planned with the Least Significant Difference (LSD) test. A p-value of 0.05 was used for all inferential statistics.
Results
The Pearson r revealed that latencies and amplitudes across Trials 1 and 2 were significantly correlated with r values ranging from 0.67 to 0.92. The mean latencies are shown in Figure 1, and the mean amplitudes are shown in Figure 2. Sample waveforms for one of the participants are presented in Figure 3. The relevant descriptive statistics are presented in Tables 1 and 2. Results of MANOVA on latencies and amplitudes are presented in Table 3.
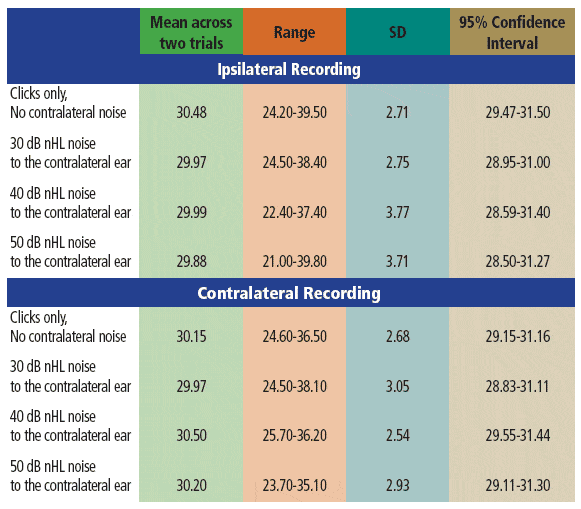
TABLE 1. Descriptive statistics for AMLR Pa latencies (ms) across the various stimulus conditions. Data across the two trials were averaged for these analyses.
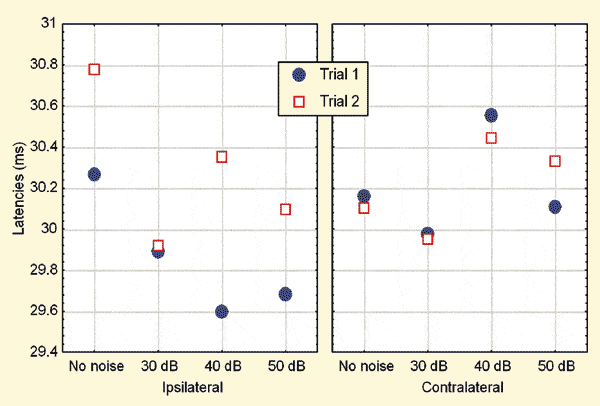
FIGURE 1. Mean latencies across various conditions. Noise levels are expressed in dB.
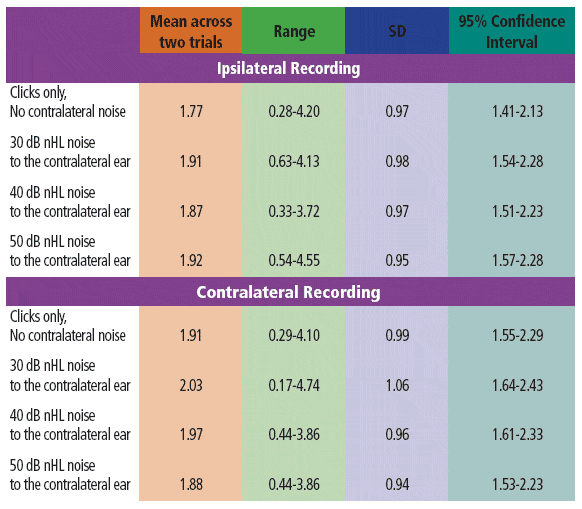
TABLE 2. Descriptive statistics for the AMLR Pa amplitudes (mV) across the various stimulus conditions. Data across the two trials were averaged for these analyses.
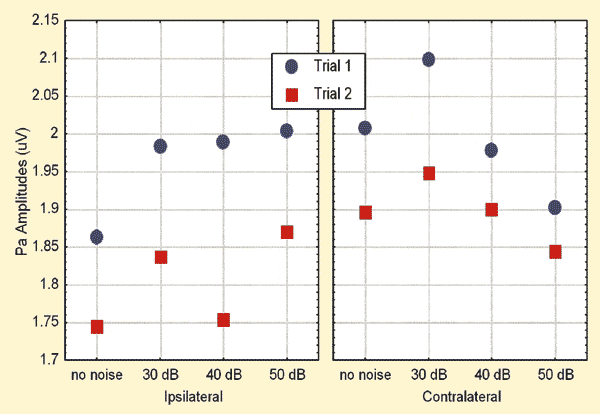
FIGURE 2. Mean amplitudes across various conditions. Noise levels are expressed in dB nHL.
As can be seen in Table 3, none of the main effects or interactions were significant at the 0.05 level. The main effect of trial missed significance for latencies (p = 0.08) and amplitudes (p = 0.10). Similarly the trial* recording mode interaction missed significance (p = 0.08) for latencies.
Due to the trend for a trial effect apparent in Figures 1 and 2, the trial effects were further explored through the LSD test. The analyses revealed that, in the ipsilateral recording mode, the latencies were significantly (p = 0.01) prolonged and amplitudes significantly (p = 0.0004) reduced during Trial 2 when compared to those in Trial 1. No such differences were apparent in the contralateral recordings.
The mean ipsilateral Pa amplitudes were larger with noise when compared to those without noise for the 30 dB and 50 dB nHL maskers. This trend was explored further by performing additional analyses with the LSD test. Results suggested significantly (p = 0.035) larger Pa amplitudes in the ipsilateral mode with the application of 30 dB nHL contralateral noise when compared to those obtained without noise. No other differences were significant.
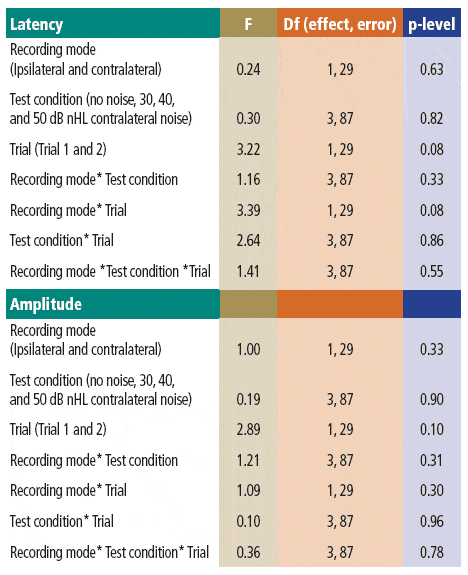
TABLE 3. Results of the MANOVAs performed on latencies and amplitudes.
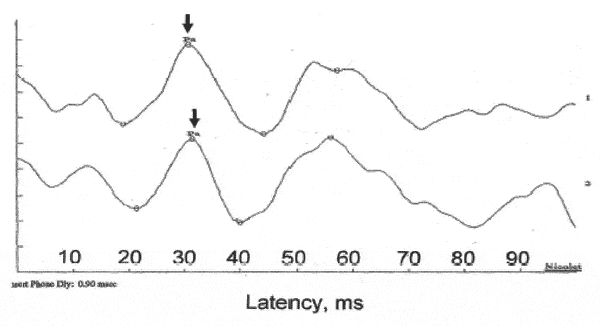
FIGURE 3. Sample AMLR waveforms for one participant. The peak of Pa is marked. Waveform A was obtained without any contralateral noise. Waveform B was obtained with 30 dBnHL contralateral noise.
Discussion
Pa latencies and amplitudes noted in the current study without contralateral noise (Tables 1 and 2) are similar to those reported by other investigators.12,49 For example, the mean ipsilateral Pa latency in the current study was 30.48 ms (SD: 2.71), while Kaseda et al12 reported mean Pa latency of 32.76 ms (SD: 3.11). Minor differences across studies can be expected due to variations in stimulus rates and levels; subject age, gender, and arousal state; and response acquisition factors.
Results presented in Tables 1 and 2 can serve as normative data for evaluation of auditory processing disorders provided that stimulus, response, and subject parameters are similar to those used in the current study.
Possible reasons for lack of suppression of Pa amplitudes with contralateral noise:
- Generally, the effect of contralateral noise on psychoacoustic auditory thresholds varies considerably among listeners. It can range from 3.5 to 12 dB for a 1 kHz gated pure-tone and a gated pure-tone masker.50 Using 40 dB SL contralateral maskers, Benton and Sheeley51 reported no threshold shifts 60% of the time and a 5 dB threshold improvement 16% of the time with wide-band maskers. This variability may obscure any main effects of contralateral masking.
- The major portion of the decrease in psychoacoustic thresholds resulting from central masking is transient. Near the onset of a pulsed masker, it may amount to 15 dB or more, but within 150 ms it is reduced to about 4 dB.52 Additionally, the central masking effect is minimal for continuous maskers.3 In the current study, a click rate of 1.1 s was used, and for each waveform 256 responses were averaged. Thus, each waveform was obtained with the continuous noise masker lasting for about 281 s. This would predict that, for a continuous masker used in the current study, the effect of central masking would be minimal. Dirks and Malmquist2 also showed that the effects of contralateral masking on psychoacoustic thresholds are smallest when the masker is continuous and the signal is pulsed.
- Nakagawa et al53 showed that AMLRs recorded with binaural clicks demonstrate some individual variability—perhaps due to attention and motivation factors with reference to the locations of sites that generate the AMLR. Results provided by their two-dipole model for the Pa component yielded three variations. In 3 of their participants, the equivalent dipole (ED) was located in each supratemporal cortex; in 3 other participants, one ED was located in the right temporal cortex and the other in the midbrain; however, in 13 participants, both EDs were located in the midbrain. Such variability may obscure any systematic suppressive effects caused by contralateral noise.
- Cortical neurons generally do not respond in a sustained fashion to continuous noise.54 This finding would predict no suppressive effects of continuous noise on the Pa component, which is probably generated in the cortical and subcortical pathways.
Possible reasons for enhancement of Pa with low level contralateral noise. In the current study, mean ipsilateral Pa amplitudes were significantly larger with 30 dB nHL noise when compared to those without noise. Goksoy and Utkucal34 suggested that the enhancement of the AMLR due to contralateral noise seen in guinea pigs might be a result of intercellular synchronization produced by shifting of the intracranial sound image from the side of the noise to the side of the click. The enhancement of ipsilateral Pa amplitudes apparent with 30 dB nHL contralateral noise observed in the current study might allow better localization of clicks in low-level noise as suggested by Goksoy and Utkucal.34
Possible reasons for a trial effect on latencies and amplitudes in the ipsilateral mode. Latencies of the ipsilateral AMLR increased during the second trial, and the amplitudes decreased. For each noise level condition, the second trial was initiated immediately after the first trial. Although we used a slow click rate of 1.1/s, which allows an interstimulus interval larger than 500 ms, the trial effect may be related to residual effects of sensory gating.
Sensory gating is usually observed by presenting paired-clicks separated by 500 msec and measuring the amplitude of the evoked potential apparent at 50 ms (P50). In normal individuals, the amplitude of the P50 component to the second click is reduced when compared to that obtained with the first click.55 This process has been termed sensory gating and is probably a subcortical process that allows the suppression of irrelevant sensory input.56 It has been suggested that multi-synaptic elements of the reticular activating system may be responsible for the sensory gating effects.57
Muller et al58 demonstrated that the sensory gating effect is already present in the Na component (the negative component before Pa) of the AMLR with a mean latency of 19 ms after the click onset. Furthermore, they noted that sensory gating is most pronounced at electrode Cz, which was the electrode location in the current study.
Through EEG, magnetoencephalography, and neuropsychological measures, Thoma et al59 showed correlations between left but not right M50 (magnetoencephalography) sensory gating ratios and behavioral measures of sustained attention in healthy participants. They also reported a correlation between P50 and left but not right M50 ratios. These results suggest that the sensory gating phenomenon may be more related to the left rather than the right hemisphere. This may predict a significant trial effect in the ipsilateral mode (left hemisphere) but not in the contralateral mode (right hemisphere) as apparent in the current study since clicks were always delivered to the left ear.
Clinical Implications
As noted in the introduction, Pa response can be used to assess hearing thresholds for lower frequencies.6,22-25 It can also be used for neuroaudiologic evaluations,23,26,27 for assessment of patients with tinnitus,28 and for assessment of auditory processing disorders.29,30
In cases of unilateral hearing loss, application of contralateral noise is necessary to minimize the participation of the normal-hearing ear. Current findings suggest no significant effect of contralateral noise on Pa amplitudes or latencies in women when the AMLR is elicited with click levels of 80 dB nHL and contralateral noise is presented at 40 or 50 dB nHL. However, the Pa amplitudes may improve upon the application of 30 dB nHL noise. The results also suggest that, when data is obtained across several trials, sufficient rest-periods should be included in between trials to minimize any possibility of sensory gating effects, which can increase Pa latencies and reduce amplitudes.
Suggestions for Future Research
Further studies will be useful in determining if results from the current study can be generalized to men and if similar or dissimilar results can be obtained when clicks are delivered to the right ear and noise is presented to the left ear.
The effects of noise on other transient auditory evoked potentials, such as steady-state evoked responses or auditory brainstem response, seem to depend on signal and masker levels.24,60 Furthermore, when stimuli at the two ears are not correlated, somewhat complex procedures are necessary to assess any binaural interactions.61 Thus, further studies are necessary to evaluate the effect of varying noise and stimulus levels on the AMLR.
References
- Wegel RL, Lane CE. The auditory masking of one pure tone by another and its probable relation to the dynamics of the inner ear. Physiol Rev. 1924;23:266-285.
- Dirks DD, Malmquist C. Shifts in air conduction thresholds produced by pulsed and continuous contralateral masking. J Acoust Soc Am. 1965;37:631-637.
- Zwislocki JJ, Buining E, Glantz J. Frequency distribution of central masking. J Acoust Soc Am. 1968;43:1267-71.
- Geisler CD, Frishkopf LS, Rosenblith WA. Extracranial responses to acoustic clicks in man. Science. 1958;128:1201-1210.
- Tucker DA, Ruth RA. Effects of age, signal level, and signal rate on the auditory middle latency response. J Am Acad Audiol. 1996;7:83-91.
- Musiek FE, Geurkink NA. Auditory brainstem and middle latency evoked response sensitivity near threshold. Ann Otol Rhinol Laryngol. 1981;90:236-240.
- Jacobson GP, Grayson AS. The normal scalp topography of the middle latency auditory evoked potential Pa component following monaural click stimulation. Brain Topogr. 1988;1:29-36
- Picton TW, Hillyard SA, Krausz HI, Galambos R. Human auditory evoked potentials. I. Evaluation of components. Electroencephalogr Clin Neurophysiol. 1974;36:179-190.
- Jacobson GP. Brain mapping of auditory evoked potentials. In: Jacobson J, ed. Principles and Applications in Auditory Evoked Potentials. Boston: Allyn and Bacon; 1994:517-40.
- Jacobson GP, Newman CW. The decomposition of the middle latency auditory evoked potential (MLAEP) Pa component into superficial and deep source contributions. Brain Topogr. 1990;2:229-236.
- Cacace AT, McFarland DJ. Middle-latency auditory evoked potentials: basic issues and potential applications. In: Katz J, ed. Handbook of Clinical Audiology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2002:349-377.
- Kaseda Y, Tobimatsu S, Morioka T, Kato M. Auditory middle-latency responses in patients with localized and non-localized lesions of the central nervous system. J Neurol. 1991;238:427-432.
- Cohen MM. Coronal topography of the middle latency auditory evoked potentials in man. Electroencephalogr Clin Neurophysiol. 1982;53:231-236.
- Borgmann C, Ross B, Draganova R, Pantev C. Human auditory middle latency responses: influence of stimulus type and intensity. Hear Res. 2001;158:57-64.
- Celesia GG, Puletti F. Auditory cortical areas of man. Neurology. 1969;19:211-220.
- Cacace AT, Satya-Murti S, Wolpaw JR. Human middle-latency auditory evoked potentials: vertex and temporal components. Electroencephalogr Clin Neurophysiol. 1990;77:6-18.
- Bell SL, Smith DC, Allen R, Lutman ME. Recording the middle latency response of the auditory evoked potential as a measure of depth of anaesthesia. A technical note. Br J Anaesthesiol. 2004;92:442-445.
- De Siena L, Pallavicino F, Lacilla M, Canale A, Longobardo A, Pecorari G, et al. Auditory-evoked potentials in general anesthesia monitoring: baseline study of availability in relation to hearing function in awake status. Acta Anaesthesiol Scand. 2005;249:774-777.
- Smith TL, Zapala D, Thompson CL, Hoye W, Kelly T. Relationship of auditory middle latency response and stem-word completion test as indicators of implicit memory formation during general anesthesia. AANA J. 1999;67:247-253.
- Fischer C, Luaute J, Adeleine P, Morlet D. Predictive value of sensory and cognitive evoked potential for awakening from coma. Neurology. 2004;63:669-673.
- Arehole S, Augustine LE, Simhadri R. Middle latency responses in children with learning disabilities: preliminary findings. J Commun Disord. 1995;28:21-38.
- Maurizi M, Ottaviani F, Paludetti G, Rosignoli M, Almadori G, Tassoni A. Middle-latency auditory components in response to clicks and low- and middle-frequency tone pips (0.5-1 kHz). Audiology. 1984;23:569-580.
- Musiek FE, Geurkink NA, Weider DJ, Donnelly K. Past, present, and future applications of the auditory middle latency response. Laryngoscope. 1984;94:1545-1553.
- Nousak JK, Stapells DR. Auditory brainstem and middle latency responses to 1 kHz tones in noise masked normally-hearing and sensorineurally hearing-impaired adults. Int J Audiol. 2005;44:331-344.
- Psillas G, Daniilidis J. Low frequency hearing assessment by middle latency responses in children with pervasive developmental disorder. Int J Ped Otorhinolaryngol. 2003;67:613-9.
- Hall JW III, Tucker DA. Sensory evoked responses in the intensive care unit. Ear Hear. 1986;7:220-232.
- Japaridze G, Shakarishvili R, Kevanishvili Z. Auditory brainstem, middle-latency, and slow cortical responses in multiple sclerosis. Acta Neurol Scand. 2002;106:47-53.
- Gerken GM, Hesse PS, Wiorkowski JJ. Auditory evoked response in control subjects and in patients with problem-tinnitus. Hear Res. 2001;157:52-64.
- Fifer RC, Sierra-Irizarry B. Clinical applications of the auditory middle latency response. Am J Otol. 1988;9(suppl):47-56.
- Purdy SC, Kelly AS, Davies MG. Auditory brainstem response, middle latency response, and late cortical evoked potentials in children with learning disabilities. J Am Acad Audiol. 2002;13:367-382.
- Ozdamar O, Kraus N, Grossmann J. Binaural interaction in the auditory middle latency response of the guinea pig. Electroencephalogr Clin Neurophysiol. 1986;63:476-483.
- Littman T, Kraus N, McGee T, Nicol T. Binaural stimulation reveals functional differences between midline and temporal components of the middle latency response in guinea pigs. Electroencephalogr Clin Neurophysiol. 1992;84:362-372.
- Kraus N, Smith DI, McGee T. Midline and temporal lobe MLRs in the guinea pig originate from different generator systems: a conceptual framework for new and existing data. Electroencephalogr Clin Neurophysiol. 1988;6:541-558.
- Goksoy C, Utkucal R. Binaural interaction component and white-noise enhancement in middle latency responses: differential effects of anaesthesia in guinea pigs. Experimental Brain Res. 2000;130:410-414.
- Goksoy C, Demitras S, Ungan P. Dynamics of the contralateral noise-induced enhancement in the guinea pig’s middle latency response. Brain Res. 2004;1017:61-68.
- Tucker DA, Dietrich S, Harris S, Pelletier S. Effects of stimulus rate and gender on the auditory middle latency response. J Am Acad Audiol. 2002;13:146-153.
- Suzuki T, Hirabayashi M. Age-related morphological changes in auditory middle-latency response. Audiology. 1987;26:312-320.
- Ali AA, Jerger J. Phase coherence of the middle-latency response in the elderly. Scand Audiol. 1992;21:187-194.
- Azumi T, Nakashima K, Takahashi K. Aging effects on auditory middle latency responses. Electromyogr Clin Neurophysiol. 1995;35:397-401.
- Chambers RD. Differential age effects for components of the adult auditory middle latency response. Hear Res. 1992;58:123-131.
- American Speech Language Hearing Association. Guidelines for screening for hearing impairment and middle-ear disorders. ASHA. 1990;32:17-32.
- Tucker DA, Dietrich S, McPherson DL, Salamat MT. Effect of stimulus intensity level on auditory middle latency response brain maps in human adults. J Am Acad Audiol. 2001;12:223-232.
- Rawool V, Zerlin S. Phase-intensity effects on the ABR. Scand Audiol. 1988;17:117-123.
- Rawool VW. Effects of click polarity on the auditory brainstem responses of older men. Audiology. 1998;37:100-108.
- Stewart MG, Jerger J, Lew HL. Effect of handedness on the middle latency auditory evoked potential. Am J Otol. 1993;14:595-600.
- Rawool VW. Acoustic reflex monitoring during the presentation of 1000 clicks at high repetition rates. Scand Audiol. 1996;25:239-245.
- Killion MC, Wilber LA, Gudmundsen GI. Insert earphones for more interaural attenuation. Hearing Instruments. 1985;36(2):34-6.
- McGee T, Kraus N, Manfredi C. Toward a strategy for analyzing the auditory middle-latency response waveform. Audiology. 1988;27:119-130.
- Erwin RJ, Buchwald JS. Midlatency auditory evoked reponses: differential recovery cycle characteristics. Electroencephalogr Clin Neurophysiol. 1986;64:417-423.
- Mills JH, Dubno JR, He N. Masking by ipsilateral and contralateral maskers. J Acoust Soc Am. 1996;100:3336-3344.
- Benton SL, Sheeley EC. Effects of three contralateral maskers on pure-tone thresholds using manual audiometry. Audiology. 1987;26:227-234.
- Zwislocki JJ, Daminopoulos EN, Buining E, Glantz J. Central masking: some steady-state and transient effects. Perception and Psychophysics. 1967;2:59-64.
- Nakagawa M, Yoshikawa H, Ando I, Ichikawa G. Equivalent dipoles for middle latency auditory evoked potentials using the dipole tracing method. Auris Nasus Larynx. 1999; 26:245-56.
- Phillips DP, Cynader MS. Some neural mechanisms in the cat’s auditory cortex underlying sensitivity to combined tone and wide-spectrum noise stimuli. Hear Res. 1985;18:87-102.
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biological Psychiatry. 1982;17:639-654.
- Hillyard SA, Kutas M. Electrophysiology of cognitive processing. Ann Rev Psychol. 1983;34:33-61.
- Buchwald JS, Erwin R, Van Lancker D, Guthrie D, Schwafel J, Tanguay P. Midlatency auditory evoked responses: P1 abnormalities in adult autistic subjects. Electromyogr Clin Neurophysiol. 1992;84:164-171.
- Muller MM, Keil A, Kissler J, Gruber T. Suppression of the auditory middle-latency response and evoked gamma-band response in a paired-click paradigm. Exp Brain Res. 2001;136:474-479.
- Thoma RJ, Hanlon FM, Moses SN, Edgar JC, Huang M, Weisend MP, et al. Lateralization of auditory sensory gating and neuropsychological dysfunction in schizophrenia. Am J Psychiatr. 2003;160:1595-1605.
- Burkard R, Hecox K. The effect of broadband noise on the human brainstem auditory evoked response. I. Rate and intensity effects. J Acoust Soc Am. 1983;74:1204-1213.
- Rawool VW, Ballachanda BB. Homo- and anti-phasic stimulation in ABR. Scand Audiol. 1990;19:9-15.
Correspondence can be addressed to Vishakha Rawool, PhD, at .
Citation for this article:
Rawool VW, Brouse MV. Effect of contralateral noise on click-evoked human auditory middle latency response. Hearing Review. 2010;17(6):24-27,50,51.





