Researchers at Indiana University School of Medicine have successfully developed a method to grow inner ear tissue from human stem cells—a finding that could lead to new platforms to model disease and new therapies for the treatment of hearing and balance disorders, the school announced.
“The inner ear is only one of few organs with which biopsy is not performed, and because of this, human inner ear tissues are scarce for research purposes,” said Eri Hashino, PhD, the Ruth C. Holton professor of otolaryngology at IU School of Medicine. “Dish-grown human inner ear tissues offer unprecedented opportunities to develop and test new therapies for various inner ear disorders.”
The study, “Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells” published online May 1, 2017, in Nature Biotechnology, was led by Karl R. Koehler, PhD, assistant professor in the Department of Otolaryngology and Head and Neck Surgery at IU School of Medicine, and Hashino in collaboration with Jeffrey Holt, PhD, professor of otology and laryngology at Harvard Medical School and Boston Children’s Hospital. The research builds on the team’s previous work with a technique called three-dimensional culture, which involves incubating stem cells in a floating ball-shaped aggregate, unlike traditional cell culture in which cells grow in a flat layer on the surface of a culture dish. This allows for more complex interactions between cells, and creates an environment that is closer to what occurs in the body during development, Koehler said. (See July 17, 2013 Hearing Review online article about this team’s previous work.)
By culturing human stem cells in this manner and treating them with specific signaling molecules, the investigators were reportedly able to guide cells through key processes involved in the development of the human inner ear. This resulted in what the scientists have termed inner ear “organoids,” or three-dimensional structures containing sensory cells and supporting cells found in the inner ear.
“This is essentially a recipe for how to make human inner ears from stem cells,” said Koehler, lead author of the study and whose research lab works on modeling human development. “After tweaking our recipe for about a year, we were shocked to discover that we could make multiple inner ear organoids in each pea-sized cell aggregate.”

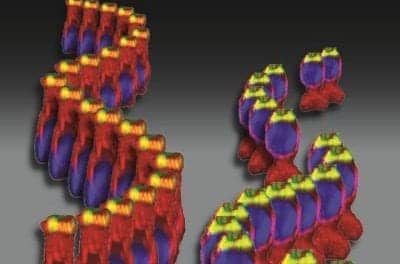
Human inner ear organoid with sensory hair cells (cyan) and sensory neurons (yellow). An antibody for the protein CTBP2 reveals cell nuclei as well as synapses between hair cells and neurons (magenta).
The researchers used CRISPR gene-editing technology to engineer stem cells that produced fluorescently-labeled inner ear sensory cells. Targeting the labeled cells for analysis, they revealed that their organoids contained a population of sensory cells that have the same functional signature as cells that detect gravity and motion in the human inner ear.
“We also found neurons, like those that transmit signals from the ear to the brain, forming connections with sensory cells,” Koehler said. “This is an exciting feature of these organoids because both cell types are critical for proper hearing and balance.”
Hashino said these findings are “a real game changer, because up until now, potential drugs or therapies have been tested on animal cells, which often behave differently from human cells.”
The researchers are currently using the human inner ear organoids to study how genes known to cause deafness interrupt normal development of the inner ear and plan to start the first-ever drug screening using human inner ear organoids.
“We hope to discover new drugs capable of helping regenerate the sound-sending hair cells in the inner ear of those who have severe hearing problems,” Hashino said.
Additional researchers on the study included Jing Nie, Emma Longworth-Mills, and Jiyoon Lee of IU School of Medicine, and Xiao-Ping Liu of Boston Children’s Hospital. The research was funded by the National Institutes of Health and the Action on Hearing Loss.
Original Paper: Koehler KR, Nie J, Longworth-Mills E, et al. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nature Biotechnology. May 1, 2017. doi: 10.1038/nbt.3840
Source: Indiana University School of Medicine, Nature Biotechnology






A great start towards the possible implantation or a natural growth process spurred by cochlear demands. What also needs to be done is to create a mash of interconnected tissue that will promote the growth of micro capillaries, to pass oxygen to the new cells, as well as pathways for nutrients, proteins and other genetic connections necessary to sustain the growth and functions.