How slower processing in the aging auditory system is related to speech recognition

|
| Vishakha W. Rawool, PhD, is a member of the faculty in the Department of Speech Pathology and Audiology at West Virginia University, Morgantown, WVa. |
This article is Part 2 of a three-part series on the aging auditory system, and it reviews the evidence for slower processing within the higher (ie, above the brainstem) auditory pathways, whereas Part 1 examined evidence of slower processing from studies that used objective measures unaffected by such things as attention or memory.1 Additionally, the present article will look at the relationship between slower processing and speech recognition. In Part 3, which will be published in next month’s HR, the complex interrelationships among slower processing, cognition and speech recognition are examined.
It has been generally established that, with aging, individuals experience a decreased ability to recognize rapid speech, even in the presence of normal hearing. The resulting communication difficulties, social isolation, and depression can contribute to an overall decrease in the quality of life.
Two of the possible underlying mechanisms for poor speech recognition in older individuals include reduced speed of signal processing within the auditory pathways of older individuals and/or reduced cognition. An in-depth understanding of this relationship may lead to provision of effective aural rehabilitation services for older individuals.
Physiological Basis for Slower Neurotransmission with Aging
Several studies have documented physiological changes within the aging auditory system that could lead to slower neurotransmission. Investigators have reported primary degeneration of the spiral ganglion (cell bodies of the auditory nerve fibers) or loss of fibers that can occur even in the absence of loss of sensory hair cells.2,3 Neuronal loss has also been reported in the cochlear nucleus, inferior colliculus, medial geniculate body, and the temporal lobe.4-6
Konigsmark and Murphy7 found a relation between age and a decrease in the volume of the cochlear nucleus that appeared to be associated with changes in axon size and degree of myelination. Degenerative changes in the myelin sheaths and axis cylinders have also been reported.5 Other degenerative changes, such as cell size and cell shape irregularities, and the possible accumulation of lipofuscin pigments, have been observed in the cochlear nucleus, superior olivary nucleus, inferior colliculus, medial geniculate body, and inferior olive.4,6,7
Based on functional MRI studies, Peelle et al8 showed that sentences presented at a rapid rate recruit frontal brain regions such as anterior cingulate and premotor cortex. These regions are also compromised in older individuals. Progressive loss of dendritic mass without obvious clinical symptoms has been reported, especially in the frontal and temporal cortex with aging.9
The above studies collectively suggest compromised or slower neurotransmission in the neural auditory pathways. Such slower processing can be expected to play a critical role in abnormal auditory perception and processing observed in older individuals.
Age-Related Transmission Delays in Central Auditory Pathways
Age-related transmission delays within the auditory pathways can be measured by using auditory evoked potentials. Birren and Fisher10 suggested that measurement of evoked potentials provides a window into the temporal nature of neural processing. More specifically, the latencies of evoked potentials can provide information on the time involved in processing the information as it travels through the various segments of the auditory pathway.
Age-related slower processing within the auditory brainstem. The auditory brainstem response (ABR) can be used to document age-related signal transmission delays within the auditory brain stem. Based on a review of literature, Rawool1 concluded that when ABRs are elicited with fairly rapid stimuli, and when the effect of age-related hearing loss is carefully controlled, the latencies and interpeak latencies are significantly prolonged with aging, suggesting slower neurotransmission within the aging auditory system. There is some variability in this effect such that not all older systems demonstrate slowing, and men may show more slowing than women.1
Age-related slower processing within the auditory thalamus and primary auditory cortex. The auditory middle latency response (AMLR) can be used to document age-related transmission delays in the thalamus and the auditory cortex. It is a far-field response that occurs between 10 and 90 ms following the onset of a transient auditory stimulus. The typical AMLR in humans can be characterized by six components: three negative (No, Na, Nb) and three positive (Po, Pa, Pb). Of all these components, Pa is considered the most constant and reliable.
In adults, the Pa component occurs approximately 20 to 40 ms following stimulus onset. When elicited with moderately high-intensity stimuli (70 to 75 dB nHL), portions of the primary auditory pathway including the temporal lobe and thalamus may contribute to the generation of the Pa waveform. Topographic studies, magnetoencephalography source analyses, and cortical recordings also suggest that Pa is generated in the auditory cortex. However, Pa may also be influenced by some deep midline brain stem generators.
Analogous to the ABR studies reviewed in Part 1 of this series,1 the effect of aging on the AMLR also appears to be controversial for similar reasons. Studies that have included only female participants have failed to show significant prolongation of Pa latencies with aging.11-13 Gender differences can be expected in the middle latency response since age-related temporal lobe atrophy is more significant in elderly men than in women.14 Gur et al15 documented age-related changes in brain volume due to brain atrophy in normal subjects ranging in age from 18 to 80 years; sulcal atrophy was significantly more pronounced in older men than in women.
Some investigators have used stimulus rates that are probably too slow (5/sec or lower) to accurately reveal age-related effects.16,17 Others have failed to match the auditory sensitivity of the younger and older individuals in the higher frequency regions.17-20 Nonetheless, a significant shift in Pa latencies reportedly occurs with age.19,20
Kelly-Ballweber and Dobie21 carefully matched the auditory sensitivity of the younger and older participants, and they reported a significant prolongation of Pa latencies with age. In conclusion, when AMLRs are generated at relatively higher rates and the auditory sensitivity is carefully matched, a significant prolongation of Pa latencies is apparent in older individuals. This suggests transmission delays within the aging thalamus and the primary temporal auditory cortex.
Age-related slower processing within the temporal lobe and adjacent parietal lobe areas. The auditory late response (ALR) can be used to evaluate age-related transmission delays within the temporal lobe and adjacent parietal lobe areas. The main components of the ALR and their characteristic latencies are P1 (50-80 ms), N1 (100-150 ms), P2 (150-200 ms), and N2 (180-250 ms). Based on existing evidence from scalp and intracranial recordings in listeners with normal hearing and temporal lobe lesions, Hall22 suggested that the generators for ALR overlap and include the posterior portion of the superior temporal plane, lateral temporal lobe, and adjacent parietal lobe areas. Several investigators have reported latency increase in the ALR with advancing age,23,24 suggesting slower processing within the temporal lobe and related parietal lobe areas.
Age-related slower processing at multiple cortical sites. The P300 is recorded as a large positive-voltage wave at approximately 300 ms in response to a rare “oddball” stimulus. Typically, rare or less frequently occurring stimuli (eg, a 500 Hz tone) are presented in the presence of frequent stimuli (eg, a 1000 Hz tone), and the participant is asked to count the rare stimuli. P300 generation involves multiple sites, including regions of the limbic system, particularly the hippocampus, mesencephalic reticular formation, medial thalamus, prefrontal cortex, frontal cortex, centroparietal cortex, and the auditory cortex. Age-related increase in P300 latencies has been noted in previous investigations.25,26
Age-related slower processing within the acoustic reflex pathway. As reviewed in Part 1,1 age-related slowing within the acoustic reflex pathway has been reported in previous studies.27,28 This suggests slower processing within the lower auditory brain stem and the efferent acoustic reflex pathways.
Age-Related Changes in Speech Recognition
A number of investigators have reported poorer speech recognition performance in older individuals on measures of speech understanding involving time-altered or fast speech.29-39
Wingfield et al40 concluded that reductions in available processing time through the use of time-compressed speech have a disproportionate effect on the comprehension-performance of the elderly participants. They assumed that time compression has its effect more from removing normally available processing time than by degrading the speech signal itself. Gordon-Salant and Fitzgibbons41 suggested that older listeners have difficulty in recognizing fast speech due to trouble in processing the brief, limited acoustic cues for consonants inherent in rapid speech.
Overall, the above studies suggest that there is an age-related decline in the rate of information processing, which makes it difficult for older listeners to understand rapid speech. Slower processing is probably at least in part related to slower neurotransmission speeds within the auditory pathways.
Slower Processing Within the Auditory Pathways and Speech Recognition
In this section, studies that suggest a relationship between slower processing or delayed neurotransmission and speech recognition are reviewed. Such a relationship suggests the possibility that speech recognition deficits experienced by older individuals may be partially caused by neurotransmission delays within the auditory pathways.
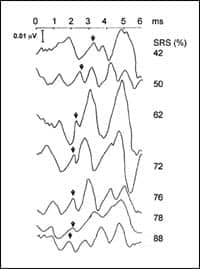
ABRs and speech recognition. Rawool42 examined the relationship between speech recognition scores in noise and the ABR in participants with hearing loss in the age range of 40 to 60 years. Participants were selected based on fairly similar audiograms but different word-recognition scores. A significant relationship was apparent between word recognition scores and latencies of waves I and II of the ABR (Figure 1). The latencies were prolonged in listeners with poor speech recognition.
The AMLR and speech recognition. Paludetti et al43 reported no relationship between speech understanding ability and AMLR in elderly people. However, the statistical bases for this conclusion were not provided and the stimulus rate for eliciting the MLR was too slow (5/sec) to sufficiently stress the aging system.
|
| FIGURE 1. Speech recognition scores (SRS) in percentage of each older individual and the corresponding ABR waveform. The arrows indicate wave II of the ABR. Note that individuals with poorer recognition scores have progressively longer latencies. The individual with the highest speech recognition score (88%) has the earliest latency for wave II.42 |
Ali and Jerger44 studied the AMLR in two groups of older listeners, with each group consisting of 10 people. Group A had speech understanding that was appropriate to the degree of hearing loss, while in Group B, speech understanding was disproportionately poor. The latency of Pa was significantly prolonged for participants in Group B when compared to those in Group A, even in the presence of somewhat better auditory sensitivity in Group B than in Group A. Thus, it appears that when AMLRs are elicited with relatively fast rates, prolongation of Pa latencies is associated with poorer speech recognition.
The ALR and speech recognition. Tremblay et al45 recorded P1, N1, and P2 components of the ALR from younger and older adults. The ALRs were evoked by synthetic speech tokens representing 10 ms increments along a /ba/-/pa/ voice-onset time (VOT) continuum. The ability to discriminate the speech tokens was also assessed. Older adults had more difficulty discriminating 10 ms VOT contrasts than younger adults. In addition, the N1 and P2 latencies were prolonged in older participants. Thus, it appears that speech recognition deficits in older adults may be partially related to the neurotransmission delays reflected in the auditory late response.
The P300 and speech recognition. Jerger et al46 compared behavioral and electrophysiological responses to verbal and nonverbal “dichotic listening” tasks in younger and older adults. The results showed an increasing left-ear deficit on verbal tasks and an increasing right-ear deficit on nonverbal tasks due to age. The P300 latency measures similarly showed the effect of age on both the verbal and nonverbal tasks.
Acoustic reflexes and speech recognition. Slower processing within the acoustic reflex pathways may be one reason for speech recognition difficulties of older individuals, at least at higher presentation levels. The reflex attenuates low-frequency components of a complex sound more than it does high-frequency components. Thus, it reduces the upward spread of masking of high-frequency sounds.47 Mahoney et al48 showed that speech recognition is better in the presence of a functioning reflex. Other investigators have demonstrated the importance of acoustic reflex in speech recognition.49
Conclusions and Implications
Poor speech recognition may be related to slower processing within the aging auditory system. Individual variability is expected in both slower processing and speech recognition deficits and is related to various factors including age, gender, and overall health of the individual.1
Depending on the particular deficits present, the most effective aural rehabilitative efforts for older individuals should include strategies to compensate for slower processing within the auditory pathways. For example, improving audibility of high frequencies through amplification may improve temporal processing in individuals with high-frequency hearing loss.50,51
Training that begins with presentation of auditory stimuli at slower rates and then progressing to stimuli that are presented at faster rates may also be helpful. Auditory training has the potential for improving neural timing in the auditory brainstem.52 Older subjects can use contextual cues effectively to overcome the difficulty in recognizing fast speech.53 Thus, training in the efficient use of contextual cues may be helpful for older individuals.
Correspondence can be addressed to HR or Vishakha Rawool at .
References
- Rawool VW. The aging auditory system, Part 1: Controversy and confusion on slower processing. Hearing Review. 2007;14(7):14-19.
- Felder E, Schrott-Fischer A. Quantitative evaluation of myelinated nerve fibres and hair cells in cochleae of humans with age-related high-tone hearing loss. Hear Res. 1995;91(1-2):19-32.
- Felix H, Johnsson LG, Gleeson M, Pollak A. Quantitative analysis of cochlear sensory cells and neuronal elements in man. Acta Otolaryngol Suppl. 1990;470:71-79.
- Brody H. An examination of cerebral cortex and brain stem aging. In: Terry RD, Gershon S, eds. Neurobiology of Aging. New York: Raven Press; 1976.
- Hansen CC, Reske-Nieslon E. Pathological studies in presbycusis. Arch Otolaryngol. 1965;82:115.
- Kirikae I, Sato S, Shitara T. A study of hearing in advanced age. Laryngoscope. 1964;74:205.
- Konigsmark BW, Murphy EA. Volume of the ventral cochlear nucleus in man: Its relationship to neuronal population and age. J Neuropathol Exp Neurol. 1972;31(2):304-316.
- Peelle JE, McMillan C, Moore P, Grossman M, Wingfield A. Dissociable patterns of brain activity during comprehension of rapid and syntactically complex speech: evidence from fMRI. Brain Lang. 2004;91(3):315-325.
- Scheibel ME, Scheibel AB. Structural changes in the aging brain. In: Brody H, Harman D, Ordy JM, eds. Aging, Vol 1. New York: Raven Press; 1975:11-37.
- Birren JE, Fisher LM. Aging and speed of behavior: Possible consequences for pscyhological functioning. Ann Rev Psychol. 1995;46:329-53.
- Azumi T, Nakashima K, Takahashi K. Aging effects on auditory middle latency response. Electromyogr Clin Neurophysiol. 1995;35:397-401.
- Chambers RD. Differential age effects for components of the adult auditory middle latency response. Hear Res. 1992;58(2):123-131.
- Chambers RD, Griffiths SK. Effects of age on the adult auditory middle latency response. Hear Res. 1991;51(1):1-10.
- Cowell PE, Turetsky BI, Gur RC, Grossman RI, Shtasel DL, Gur RE. Sex differences in aging of the human frontal and temporal lobes. J Neurosci. 1994;14:4748-4755.
- Gur RC, Mozley PD, Resnick SM, et al. Gender differences in age effect on brain atrophy measured by magnetic resonance imaging. Proc Natl Acad Sci USA. 1991;88:2845-2849.
- Paludetti G, Maurizi M, D’Alatri L, Galli J. Relationships between middle latency auditory responses (MLR) and speech discrimination tests in the elderly. Acta Otolaryngol (Stockh) Suppl. 1991;476:105-9.
- Amenedo E, Diaz F. Effects of aging on middle-latency auditory evoked potentials: A cross-sectional study. Biol Psychiatry. 1998;43(3):210-129.
- Martini A, Comacchio F, Magnavita V. Auditory evoked responses (ABR, MLR, SVR) and brain mapping in the elderly. Acta Otolaryngol (Stockh) Suppl. 1990;476:97-103.
- Woods DL, Clayworth CC. Age-related changes in human middle latency auditory evoked potentials. Electroencephalogr Clin Neurophysiol. 1986;65(4):297-303.
- Lenzi A, Chiarelli G, Sambataro G. Comparative study of middle latency responses and auditory brainstem responses in elderly subjects. Audiology. 1989;28(3):144-151.
- Kelly-Ballweber D, Dobie RA. Binaural interaction measured behaviorally and electrophysiolgically in young and old adults. Audiology. 1984;23(2):181-194.
- Hall JW III. Handbook of Auditory Evoked Responses. Boston: Allyn and Bacon; 1992.
- Pfefferbaum A, Ford JM, Roth WT, Kopell BS. Age-related changes in auditory event-related potentials. Electroencephalogr Clin Neurophysiol. 1980;49(3-4):266-276.
- Goodin DS, Squires KC, Henderson BH, Starr A. Age-related variations in evoked potentials to auditory stimuli in normal human subjects. Electroencephalogr Clin Neurophysiol. 1978;44(4):447-458.
- Polich JM, Howard L, Starr A. Effects of age on the P300 component of the event-related potential from auditory stimuli: Peak definition, variation, and measurement. J Gerontol. 1985;40:721-726.
- Iragui VJ, Kutas M, Mitchiner MR, Hillyard SA. Effects of aging on even-related brain potentials and reaction times in an auditory oddball task. Psychophysiology. 1993;30(1):10-22.
- Bosatra A, Russolo M, Silverman CA. Acoustic reflex latency: State of the art. In: Silman S, ed. The Acoustic Reflex: Basic Principles and Clinical Applications. Burlington, Mass: Academic Press Inc; 1984:301-328.
- Rawool VW. Effect of aging on the click-rate induced facilitation of the acoustic reflex thresholds. J Gerontol Biol Sci. 1996;51A:B124-131.
- Bergman M, Blumenfeld VG, Cascardo D, Dash B, Levitt H, Margulies MK. Age-related decrement in hearing for speech. J Gerontol. 1976;31(6):533-538.
- Konkle DF, Beasley DS, Bess FH. Intelligibility of time-altered speech in relation to chronological aging. J Speech Hear Res. 1977;20:108-115.
- Letowski T, Poch N. Comprehension of time-compressed speech: Effects of age and speech complexity. J Am Acad Audiol. 1996;7(6):447-457.
- Schmitt JF, Carroll MR. Older listeners’ ability to comprehend speaker generated rate alteration of passages. J Speech Hear Res. 1985;28(2):309-312.
- Sticht TG, Gray BB. The intelligibility of time compressed words as a function of age and hearing loss. J Speech Hear Res. 1969;12(2):443-448.
- Stine EL, Wingfield A., Poon LW. How much and how fast: rapid processing of spoken language in later adulthood. Psychol Aging. 1986;1(4):303-311.
- Gordon-Salant S, Fitzgibbons PJ. Temporal factors and speech recognition performance in young and elderly listeners. J Speech Lang Hear Res. 1993;36(6):1276-1285.
- Gordon-Salant S, Fitzgibbons PJ. Recognition of multiply degraded speech by young and elderly listeners. J Spch Lang Hear Res. 1995;38(6):1150-1156.
- Gordon-Salant S, Fitzgibbons PJ. Profile of auditory temporal processing in older listeners. J Speech Lang Hear Res. 1999;42(2):300-311.
- Gordon-Salant S, Fitzgibbons PJ. Effects of stimulus and noise rate variability on speech perception by younger and older adults. J Acoust Soc Am. 2004;115(4):1808-17.
- Versfeld NJ, Dreschler WA. The relationship between the intelligibility of time-compressed speech and speech in noise in young and elderly listeners. J Acoust Soc Am. 2002;111(1 Pt 1):401-408.
- Wingfield A, Poon LW, Lombardi L, Lowe D. Speed of processing in normal aging: effects of speech rate, linguistic structure, and processing time. J Gerontol. 1985;40(5):579-585.
- Gordon-Salant S, Fitzgibbons PJ. Sources of age-related recognition difficulty for time-compressed speech. J Speech Lang Hear Res. 2001;44(4):709-19.
- Rawool VW. Speech recognition scores and ABR in cochlear impairment. Scand Audiol. 1989;18(2):1-5.
- Paludetti G, Maurizi M, D’Alatri L, Galli J. Relationships between middle latency auditory responses (MLR) and speech discrimination tests in the elderly. Acta Otolaryngol (Stockh) Suppl. 1991;476:105-109.
- Ali AA, Jerger J. Phase coherence of the middle latency response in the elderly. Scand Audiol. 1992;21(3):187-194.
- Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol. 2003;114(7):1332-1343.
- Jerger J, Alford B, Lew H, Rivera V, Chmiel R. Dichotic listening, event-related potentials, and interhemispheric transfer in the elderly. Ear Hear. 1995;16(5):482-498.
- Borg E, Counter A. The middle-ear muscles. Scientific American. 1989;August:74-80.
- Mahoney T, Vernon J, Meikle M. Function of the acoustic reflex in discrimination of intense speech. Arch Otolaryngol. 1979;105(3):119-23.
- Colletti V, Fiorino FG, Verlato G, Carner M. Acoustic Reflex in Frequency Selectivity: Brain stem auditory evoked response and speech discrimination. In: Katz J, Stecker NA, Henderson D. Central Auditory Processing: A Transdisciplinary View. St. Louis: Mosby Year Book; 1992:39-46.
- Bacon SP, Gleitman RM. Modulation detection in subjects with relatively flat hearing losses. J Speech Hear Res. 1992;35(3):642-653.
- Bacon SP, Viemeister NF. Temporal modulation transfer functions in normal-hearing and hearing-impaired subjects. Audiology. 1985;24(2):117-134.
- Russo NM, Nicol TG, Zecker SG, Hayes EA, Kraus N. Audiory training improves neural timing in the human brainstem. Behav Brain Res. 2005;156(1):95-103.
- Wingfield A, Poon LW, Lombardi L, Lowe D. Speed of processing in normal aging: Effects of speech rate, Linguistic structure and processing time. J Gerontol. 1985;40(5):579-585.