Research scientists at Boston Children’s Hospital and Harvard Medical School have used gene therapy to restore hearing in mice with a genetic form of deafness. Their work, which is described in an online article in the July 8, 2015 edition of Science Translational Medicine, could pave the way for gene therapy in people with hearing loss caused by genetic mutations.
“Our gene therapy protocol is not yet ready for clinical trials—we need to tweak it a bit more—but in the not-too-distant future we think it could be developed for therapeutic use in humans,” said Jeffrey Holt, PhD, a scientist in the Department of Otolaryngology and F.M. Kirby Neurobiology Center at Boston Children’s, and an associate professor of otolaryngology at Harvard Medical School.
According to an announcement from Boston Children’s Hospital, more than 70 different genes are known to cause deafness when mutated. Severe to profound congenital hearing loss in both ears affects 1 to 3 per 1,000 live births. For this study, Holt and first author Charles Askew and colleagues at École Polytechnique Fédérale de Lausanne in Switzerland focused on a gene called TMC1. They chose TMC1 because it is a common cause of genetic deafness, accounting for 4-8% of cases, and encodes a protein that plays a central role in hearing, helping convert sound into electrical signals that travel to the brain.

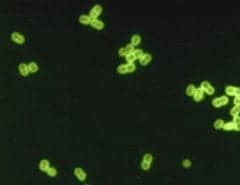
Sensory hair cells in the cochlea of a Beethoven mouse treated with TMC2 gene therapy. In this confocal microscopy image, microvilli are shown in red and cell bodies in green. (Photo: Courtesy of Charles Askew)
The researchers reportedly tested gene therapy in two types of mutant mice. One type had the TMC1 gene completely deleted, and is a good model for recessive TMC1 mutations in humans: children with two mutant copies of TMC1 have profound hearing loss from a very young age, usually by around 2 years. The other type of mouse, called Beethoven, has a specific TMC1 mutation—a change in a single amino acid—and is a good model for the dominant form of TMC1-related deafness. In this form, less common than the recessive form, a single copy of the mutation causes children to gradually go deaf beginning around the age of 10 to 15 years.
To deliver the healthy gene, the team inserted it into an engineered virus called adeno-associated virus 1, or AAV1, together with a promoter—a genetic sequence that turns the gene on only in certain sensory cells of the inner ear known as hair cells. They then injected the gene-bearing AAV1 into the inner ear.
According to the study authors, their experiments produced several findings. In the recessive deafness model, gene therapy with TMC1 restored the ability of sensory hair cells to respond to sound—producing a measurable electrical current—and also restored activity in the auditory portion of the brainstem. The authors report that the deaf mice regained their ability to hear. To test hearing, the researchers placed the mice in a “startle box” and sounded abrupt, loud tones. In the dominant deafness model, gene therapy with a related gene, TMC2, was successful at the cellular and brain level, and partially successful at restoring actual hearing in the startle test.
Clinical Trials of Gene Therapy for Congenital Deafness Anticipated in 5-10 Years
The research team reports that AAV1 is considered safe as a viral vector and is already in use in human gene therapy trials for blindness, heart disease, muscular dystrophy and other conditions. They screened various types of AAV and various types of promoters to choose the best-performing combination, and plan to further optimize their protocol and follow their treated mice to see if they retain hearing longer than the two months already observed.
Ultimately, Holt hopes to partner with clinicians at Boston Children’s Department of Otolaryngology and elsewhere to start clinical trials of TMC1 gene therapy within 5 to 10 years.
“Current therapies for profound hearing loss like that caused by the recessive form of TMC1 are hearing aids and cochlear implants,” said Margaret Kenna, MD, MPH, a specialist in genetic hearing loss at Boston Children’s Hospital who is familiar with the work. “Cochlear implants are great, but your own hearing is better in terms of range of frequencies, nuance for hearing voices, music and background noise, and figuring out which direction a sound is coming from. Anything that could stabilize or improve native hearing at an early age is really exciting and would give a huge boost to a child’s ability to learn and use spoken language.”
According to Holt, other forms of genetic deafness may also be amenable to the same gene therapy strategy. “I can envision patients with deafness having their genome sequenced, and a tailored, precision medicine treatment injected into their ears to restore hearing,” Holt says.
For more details about the study, read the announcement from Boston Children’s Hospital and the article in Science Translational Medicine.
Source: Boston Children’s Hospital
Photo credits: Charles Askew, École Polytechnique Fédérale de Lausanne; Children’s Hospital; Harvard Medical School; University of Virginia