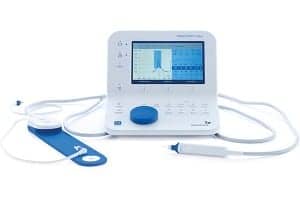
GN Otometrics, a global manufacturer of audiology instrumentation and software solutions, announced that the new MADSEN® Zodiac—its newest immittance testing solution—was recently cleared by the US Food and Drug Administration (FDA). The new solution, built from the ground up with special focus on the probes, is intended to bring greater control, confidence, and efficiency to immittance testing.
Distinctive Probe Design with Dual Probe™ Option
The new MADSEN Zodiac is designed with particular focus on the probes and the way clinicians work during immittance testing. There are three probes – Quick Check for screening and Classic or Comfort diagnostic probes for advanced testing. The probes are designed to be ergonomic, easy to use, and lightweight, helping clinicians achieve faster and more stable placement and a reliable seal.
MADSEN Zodiac is said to be the only immittance testing solution with a dual probe option. This allows the clinician to keep the screening probe and the diagnostic probe connected at the same time – all the time – ready to use for uninterrupted workflow. In addition, light and audio indicators on the probes provide immediate information about the seal, which ear you are testing and when the test starts and stops. Remote controls on the probes and shoulder strap allow clinicians to stay close to the patient throughout the test.
Streamlined Immittance Screening and Testing
The immittance tester has a dedicated immittance module based on OTOsuite® software – the shared integrated interface for all Otometrics hearing assessment and fitting solutions. A flat menu structure and easy to use software makes it easy to work in the system without getting lost. Clinicians can work with immittance, assessment, OAE, video otoscopy, and fitting in one easy to use system. Flexible data handling enables you to combine data from one or all of your devices and create professional, customized reports.
MADSEN Zodiac is available in three versions: Quick Check, Diagnostic, and Clinical, and as stand-alone or PC-based solution, with the PC-based solution optimized for touch screen. For more information about MADSEN Zodiac, visit the Audiology Systems website.
GN Otometrics (Otometrics) provides instrumentation and software solutions to hearing and balance care professionals worldwide. For more than 50 years, Otometrics has been helping hearing care professionals improve the quality of life for patients around the world by delivering reliable, innovative solutions, and expert knowledge and training. Otometrics develops, manufactures and markets computer-based audiological, otoneurologic and vestibular instrumentation in more than 70 countries under the MADSEN®, AURICAL®, HORTMANN®, ICS® and GENIE™ brand names. Otometrics is part of the GN Group, one of the largest companies in Denmark.
Audiology Systems is the official US distributor of Otometrics products: MADSEN, AURICAL, ICS and GENIE sound rooms. Audiology Systems was founded to elevate customer care through increased professionalism, consultative dialogue, and accessibility to customers.
Source: GN Otometrics; Audiology Systems